Hydrophobicity-dependent effects of polymers on different protein conformations
Abstract
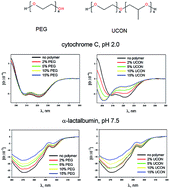
We have previously shown that increasing the hydrophobicity of PEG by adding a methyl group to every other monomer unit allowed the resulting polymer to alter protein folding and inhibit protein aggregation to amyloid fibrils. As a continuation of this work, we analyzed here the effects of this substitution on the structural properties of proteins capable of adopting multiple conformations (folded, and different partially folded states, e.g. a molten globule-like intermediate) at mild denaturing conditions. To this end, we have selected several proteins (α-lactalbumin, apomyoglobin, carbonic anhydrase, staphylococcal nuclease, and cytochrome c) and examined them at different conditions where they exist in different partially folded conformations (pH, temperature, salt concentrations, presence of cofactors). We were especially interested in the relative sensitivity of partially folded (e.g. molten globule) conformations of these proteins to the presence of polymers as these conformations are often the most sensitive to the environment. We used far-UV CD to test the changes in the protein secondary structure, near-UV CD to monitor changes in the tertiary structure, and quenching of intrinsic protein fluorescence by acrylamide to evaluate changes in the solvent accessibility of aromatic residues. We found that the complexity of the effect of polymers on protein structure cannot be ascribed solely to macromolecular crowding since the behavior of proteins in solutions containing polymers is dependent on protein and polymer structure. We also cannot exclude the possibility that the structures of both proteins and polymers determine the balance between attractive and repulsive forces that drive protein–polymer interactions.

 Please wait while we load your content...
Please wait while we load your content...