Synthesis and pH-responsive self-assembly behavior of a fluorescent amphiphilic triblock copolymer mPEG-b-PCL-b-PDMAEMA-g-PC for the controlled intracellular delivery of doxorubicin
Abstract
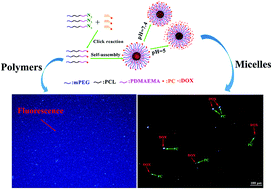
In this work, well-defined pH-responsive methoxy poly(ethylene glycol)-block-poly(ε-caprolactone)-block-poly[2-(dimethylamino)ethyl methacrylate]-g-7-propinyloxy coumarin triblock amphiphilic copolymers (mPEG-b-PCL-b-PDMAEMA-g-PC) were synthesized using a combination of atom transfer radical polymerization (ATRP), ring opening polymerization (ROP) and click chemistry. The chemical structures and compositions of these copolymers were characterized using Fourier transform infrared spectroscopy (FT-IR) and proton nuclear magnetic resonance (1H NMR). The molecular weights of the copolymers were obtained using 1H NMR spectroscopy and gel permeation chromatography (GPC) measurements. Subsequently, the polymers could self-assemble into micelles which were investigated using dynamic light scattering (DLS), transmission electron microscopy (TEM), and fluorescence spectroscopy. The pH-responsive self-assembly behavior of these triblock copolymers in water were investigated at different pH values of 5 and 7.4 for controlled doxorubicin release, the results indicated that the release rate of DOX could be effectively controlled by altering the pH, DOX was sealed in neutral surroundings and DOX release was triggered in acidic surroundings. CCK-8 assays and confocal laser scanning microscopy (CLSM) against HeLa cells indicated that the micelles had no associated cytotoxicity, possessed good biodegradability and biocompatibility, and identified the location of the DOX in HeLa cells. The DOX-loaded micelles possessed high cytotoxicity to HeLa cells and exhibited inhibition of the proliferation of HeLa cells. Moreover, these flexible micelles with an on–off switched drug release may offer a promising pattern to deliver a wide variety of hydrophobic payloads to tumor cells for cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...