The impact of α-hydrazino acids embedded in short fluorescent peptides on peptide interactions with DNA and RNA†
Abstract
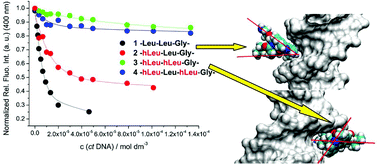
A series of novel hydrazino-based peptidomimetics and analogues comprising N-terminal lysine and C-terminal phenanthridinyl-L-alanine were prepared. The presented results demonstrate the up to now unknown possibility to finely modulate peptide interactions with DNA/RNA by α-hydrazino group insertion and how the different positioning of two α-hydrazino groups in peptides controls binding to various double stranded and single stranded DNA and RNA. All peptidomimetics bind with 1–10 micromolar affinity to ds-DNA/RNA, whereby the binding mode is a combination of electrostatic interactions and hydrophobic interactions within DNA/RNA grooves. Insertion of the α-hydrazino group into the peptide systematically decreased its fluorimetric response to DNA/RNA binding in the order: mono-hydrazino < alternating-hydrazino < sequential-hydrazino group. Binding studies of ss-polynucleotides suggest intercalation of phenanthridine between polynucleotide bases, whereby affinity and fluorimetric response decrease with the number of α-hydrazino groups in the peptide sequence. Particularly interesting was the interaction of two sequential α-hydrazino acids-peptidomimetic with poly rG, characterised by a specific strong increase of CD bands, while all other peptide/ssRNA combinations gave only a CD-band decrease. All mentioned interactions could also be reversibly controlled by adjusting the pH, due to the protonation of the fluorophore.

 Please wait while we load your content...
Please wait while we load your content...