Benzimidazole covalent probes and the gastric H+/K+-ATPase as a model system for protein labeling in a copper-free setting
Abstract
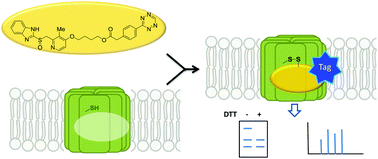
Affinity probes are useful tools for determining molecular targets and elucidating mechanism of action for novel, bioactive compounds. In the case of covalent inhibitors, activity based probes are particularly valuable for ensuring acceptable selectivity margins. However, there is a variety of bioorthogonal chemistry reactions available for modifying compounds of interest with clickable tags. Here, we describe a direct comparison of tetrazine ligation and strain promoted azide–alkyne cycloaddition using benzimidazole based probes to bind their known target, the gastric proton pump, ATP4A. This study validates the use of chemical probes for target identification and illustrates the superior efficiency of tetrazine ligation for copper-free click systems. In addition, we have identified several novel binding partners of benzimidazole probes: Isoform 2 of deleted in malignant brain tumors 1 protein (DMBT1) and three uncharacterized proteins.
- This article is part of the themed collections: Chemical Biology in Molecular BioSystems and Protein Labelling

 Please wait while we load your content...
Please wait while we load your content...