Group 6 metal pentacarbonyl complexes of air-stable primary, secondary, and tertiary ferrocenylethylphosphines†
Abstract
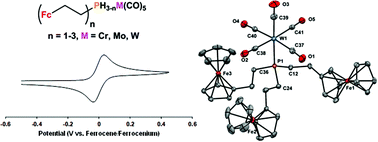
The synthesis and characterization of a series of Group 6 metal pentacarbonyl complexes of air stable primary, secondary, and tertiary phosphines containing ferrocenylethyl substituents are reported [M(CO)5L: M = Cr, Mo, W; L = PH2(CH2CH2Fc), PH(CH2CH2Fc)2, P(CH2CH2Fc)3]. The structure and composition of the complexes were confirmed by multinuclear NMR spectroscopy, IR and UV-Vis absorption spectroscopy, mass spectrometry, X-ray crystallography, and elemental analysis. The solid-state structural data reported revealed trends in M–C and M–P bond lengths that mirrored those of the atomic radii of the Group 6 metals involved. UV-Vis absorption spectroscopy and cyclic voltammetry highlighted characteristics consistent with electronically isolated ferrocene units including wavelengths of maximum absorption between 435 and 441 nm and reversible one-electron (per ferrocene unit) oxidation waves between 10 and −5 mV relative to the ferrocene/ferrocenium redox couple. IR spectroscopy confirmed that the σ donating ability of the phosphines increased as ferrocenylethyl substituents were introduced and that the tertiary phosphine ligand described is a stronger σ donor than PPh3 and a weaker σ donor than PEt3, respectively.

 Please wait while we load your content...
Please wait while we load your content...