A mechanistic model for hydrogen activation, spillover, and its chemical reaction in a zeolite-encapsulated Pt catalyst†
Abstract
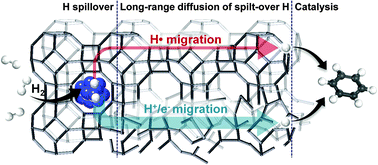
The hydrogen (H) spillover phenomenon has attracted considerable attention in the catalysis field. Many researchers have focused on the phenomenon itself, as well as its applications for advanced catalytic systems. In particular, H spillover on non-reducible materials, such as alumina, silica, and zeolites, is a controversial issue owing to the lack of understanding regarding its mechanistic properties. In this study, we use density functional theory calculations to propose the entire mechanism of H spillover from H2 activation on a platinum to its participation in chemical reactions on the external surface of a zeolite. We determined that surface hydroxyl groups of the zeolites, such as Brønsted acid sites, play a role in initiating H spillover, and the Lewis acid sites facilitate the entire process by allowing H to be transferred as a H+/e− charge pair, as well as providing good binding sites for organic reactants. Theoretical results explain the key experimental features, and we expect that this work will help to elucidate the H spillover phenomenon on non-reducible support materials and to utilize it for catalytic systems.

 Please wait while we load your content...
Please wait while we load your content...