Theoretical rationalisation for the mechanism of N-heterocyclic carbene-halide reductive elimination at CuIII, AgIII and AuIII†
Abstract
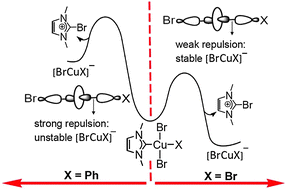
Reductive elimination of imidazolium salts from CuIII is extremely sensitive to the anionic ligand (X or Y) type on Cu (e.g. ΔG‡ ranges from 4.7 kcal mol−1 to 31.8 kcal mol−1, from chloride to benzyl). Weakly σ-donating ligands dramatically accelerate reductive elimination. Comparison with Ag/Au shows that the HOMO energy, strength of M–NHC and M–Y bonds and inherent stability of MIII with respect to MI are critical to governing reaction feasibility.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...