Synthesis and characterization of a novel N–F reagent derived from the ethano-Tröger's base: 1JFN coupling constants as a signature for the N–F bond†
Abstract
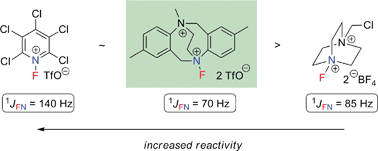
Methylation of 2,8-dimethyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]-diazocine (ethano-Tröger's base) with methyl iodide followed by ion metathesis and fluorination with N-fluoro-2,3,4,5,6-pentachloropyridinium triflate affords a new electrophilic N–F reagent, that is more reactive than Selectfluor. 2D 19F–15N HMQC experiments provide 1JNF coupling constants which are diagnostic for the N–F functional group.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...