Electrocatalytic hydrogen evolution using graphitic carbon nitride coupled with nanoporous graphene co-doped by S and Se†
Abstract
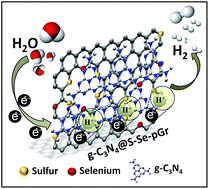
Electrocatalytic hydrogen evolution using non-precious metals or metal-free catalysts is critically necessary because platinum-based electrocatalysts are greatly limited in scalable commercialization of hydrogen generation due to their high cost. Here, we report the facile synthesis of metal-free hybrid catalysts, in which graphitic carbon nitride (g-C3N4) is coupled with nanoporous graphene doped by S and Se. The S and Se co-doped hybrid catalyst (g-C3N4@S–Se-pGr) reveals superior electrocatalytic performances, including an exchange current density of 6.27 × 10−6 A cm−2, an on-set potential of 0.092 V, a Tafel slope of 86 mV dec−1, an adsorption free energy of −0.13 eV, and long-term stability comparable to those of commercial Pt/C catalysts. Volcano plots showing the hydrogen evolution activity versus adsorption free energy are also compatible with those of the conventional metal catalysts. Our strategy has the potential to allow a new paradigm for the development of high-performance metal-free electrocatalysts for energy conversion devices.

 Please wait while we load your content...
Please wait while we load your content...