A cell-penetrating protein designed for bimodal fluorescence and magnetic resonance imaging†
Abstract
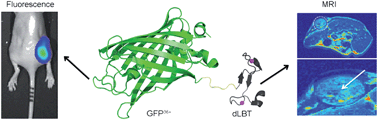
Multimodal imaging is a highly desirable biomedical application since it can provide complementary information from each imaging modality. We propose a protein engineering-based strategy for the construction of a bimodal probe for fluorescence and magnetic resonance imaging. A recombinant protein was generated by the fusion of a supercharged green fluorescence protein (GFP36+) with a lanthanide-binding tag (dLBT) that can stably bind two Gd3+ ions. The GFP36+–dLBT fusion protein showed strong fluorescence and exhibited efficient contrast enhancement in magnetic resonance imaging. This protein probe improves the MR relaxation more efficiently than Gd-DTPA (gadopentetate dimeglumine). The superior cell-penetrating activity of GFP36+ allows the efficient cellular uptake of this fusion protein and it can thus be used as a cellular imaging probe. Dual imaging was conducted in vitro and in mice. This result indicates that the fusion of different functional domains is a feasible approach for making multi-modal imaging agents.
- This article is part of the themed collection: Global challenges: Health & Food

 Please wait while we load your content...
Please wait while we load your content...