Highly stable nano Ce–La catalyst for hydrogen production from bio-ethanol
Abstract
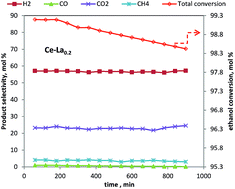
The catalytic activity of a metal free nano particle, whose size ranged from 1–10 nm, was studied according to HRTEM and DLS results. The effect of reaction temperature (300–600 °C) on the catalytic activity of the prepared catalysts was studied in a flow system under atmospheric pressure with an ethanol–water molar ratio of 1 : 10. Selectivity was calculated for the products H2, CO, CO2 and CH4, as well as the intermediates C2H6, C2H4, CH3CHO and CH3COCH3, at different reaction temperatures. It was found that the addition of La increased the activity and stability of the Ce catalyst. H2 was produced at the lowest temperature (300 °C) over Ce–La0.2 & Ce–La0.5 catalysts. Complete conversion of ethanol with high hydrogen selectivity was obtained at 500 °C over Ce–La0.2 catalysts. Further increases of La content lead to decrease in the catalyst activity in accordance with the decrease of surface area to 9.6 m2 g−1. To study the stability of the prepared catalyst, the ESR reactions of Ce–La0.2 and Ce–La1 catalysts were investigated at 500 °C for 15 h. It was found that the Ce–La0.2 catalyst is more stable than the Ce–La1 catalyst over entire 15 h. Finally, we can say that lanthanum oxide (La2O3) is particularly suitable as a modifier due to its effectiveness in the prevention of sintering and improvement of thermal resistance at high temperatures. This oxide is also able to gasify the coke through the formation of La2O2CO3 oxy-carbonate.

 Please wait while we load your content...
Please wait while we load your content...