DMSO/I2 mediated C–C bond cleavage of α-ketoaldehydes followed by C–O bond formation: a metal-free approach for one-pot esterification†
Abstract
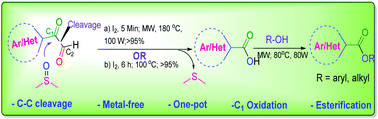
A novel and efficient I2/DMSO mediated metal-free strategy is presented for the direct C–C bond cleavage of aryl-/heteroaryl- or aliphatic α-ketoaldehydes by C2-decarbonylation and C1-carbonyl oxidation to give the corresponding carboxylic acids followed by esterification in one pot, offering excellent yields in both the steps. Here, DMSO acts as the oxygen source/oxidant and this reaction works very well under both conventional heating and microwave irradiation. This is a very simple and convenient protocol.

 Please wait while we load your content...
Please wait while we load your content...