Phosphoribulokinase from Chlamydomonas reinhardtii: a Benson–Calvin cycle enzyme enslaved to its cysteine residues
Abstract
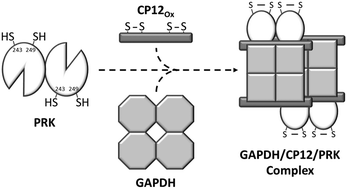
Phosphoribulokinase (PRK) in the green alga Chlamydomonas reinhardtii is a finely regulated and well-studied enzyme of the Benson–Calvin cycle. PRK can form a complex with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the small chloroplast protein CP12. This study aimed to determine the molecular determinants on PRK involved in the complex and the mechanism of action of a recently described novel regulation of PRK that involves glutathionylation. A combination of mass spectrometry, mutagenesis and activity analyses showed that Cys16, besides its role as the binding site of ATP, was also the site for S-glutathionylation. Previous kinetic analysis of the C55S mutant showed that in the oxidized inactive form of PRK, this residue formed a disulfide bridge with the Cys16 residue. This is the only bridge reported for PRK in the literature. Our data show for the first time that a disulfide bridge between Cys243 and Cys249 on PRK is required to form the PRK–GAPDH–CP12 complex. These results uncover a new mechanism for the PRK–GAPDH–CP12 formation involving a thiol disulfide exchange reaction with CP12 and identify Cys16 of PRK as a target of glutathionylation acting against oxidative stress. Although Cys16 is the key residue involved in binding ATP and acting as a defense against oxidative damage, the formation of the algal ternary complex requires the formation of another disulfide bridge on PRK involving Cys243 and Cys249.

 Please wait while we load your content...
Please wait while we load your content...