Elucidating the structure of the magnesium aluminum chloride complex electrolyte for magnesium-ion batteries†
Abstract
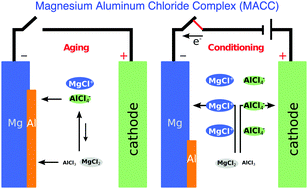
Non-aqueous Mg-ion batteries offer a promising way to overcome safety, costs, and energy density limitations of state-of-the-art Li-ion battery technology. We present a rigorous analysis of the magnesium aluminum chloride complex (MACC) in tetrahydrofuran (THF), one of the few electrolytes that can reversibly plate and strip Mg. We use ab initio calculations and classical molecular dynamics simulations to interrogate the MACC electrolyte composition with the goal of addressing two urgent questions that have puzzled battery researchers: (i) the functional species of the electrolyte, and (ii) the complex equilibria regulating the MACC speciation after prolonged electrochemical cycling, a process termed as conditioning, and after prolonged inactivity, a process called aging. A general computational strategy to untangle the complex structure of electrolytes, ionic liquids and other liquid media is presented. The analysis of formation energies and grand-potential phase diagrams of Mg–Al–Cl–THF suggests that the MACC electrolyte bears a simple chemical structure with few simple constituents, namely the electro-active species MgCl+ and AlCl4− in equilibrium with MgCl2 and AlCl3. Knowledge of the stable species of the MACC electrolyte allows us to determine the most important equilibria occurring during electrochemical cycling. We observe that Al deposition is always preferred to Mg deposition, explaining why freshly synthesized MACC cannot operate and needs to undergo preparatory conditioning. Similarly, we suggest that aluminum displacement and depletion from the solution upon electrolyte resting (along with continuous MgCl2 regeneration) represents one of the causes of electrolyte aging. Finally, we compute the NMR shifts from shielding tensors of selected molecules and ions providing fingerprints to guide future experimental investigations.


 Please wait while we load your content...
Please wait while we load your content...