Insights into the denaturation of bovine serum albumin with a thermo-responsive ionic liquid†
Abstract
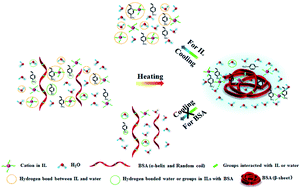
Influence of bovine serum albumin on the phase transition behavior of the synthetic ionic liquid tetrabutylphosphonium styrenesulfonate ([P4,4,4,4][SS]) together with the interactions between [P4,4,4,4][SS] and bovine serum albumin (BSA) was investigated by differential scanning calorimetry (DSC), turbidity measurements, FT-IR, in combination with perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2DCOS). Our results reveal that the addition of BSA would increase the phase transition temperature but weaken the transition behavior of [P4,4,4,4][SS] solution. DSC and turbidity data show us that the transition temperature of a ternary system with 20 wt% BSA added is 3 °C higher than that with 20% (w/v) [P4,4,4,4][SS] solution. Interactions between [P4,4,4,4][SS] and BSA together with the phase transition behavior of [P4,4,4,4][SS] are responsible for the denaturation of BSA upon heating. PCMW determined the obvious distinctions in LCST of different chemical groups manifesting their various response sequences in the phase separation and denaturation upon heating. Finally, 2DCOS was employed to elucidate the sequential order of chemical group motions during heating. It is worth noting that the appearance of the isosbestic point in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of FTIR spectra indicates the direct transformation of the conformation of α-helix, random coil to β-sheet and β-turn without an intermediate transition state. Additionally, the phase separation process of ionic liquid is able to recover to the original state before heating while the denaturation of BSA is irreversible after a cooling process.
O groups of FTIR spectra indicates the direct transformation of the conformation of α-helix, random coil to β-sheet and β-turn without an intermediate transition state. Additionally, the phase separation process of ionic liquid is able to recover to the original state before heating while the denaturation of BSA is irreversible after a cooling process.

 Please wait while we load your content...
Please wait while we load your content...