LCST transition of PNIPAM-b-PVCL in water: cooperative aggregation of two distinct thermally responsive segments†
Abstract
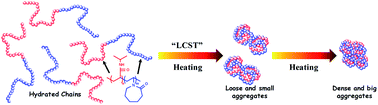
The self-aggregation behavior of poly(N-isopropylacrylamide)-b-poly(N-vinylcaprolactam) (PNIPAM-b-PVCL) during the thermal-induced phase transition in water was explored by a combination of calorimetric, turbidity, dynamic light scattering (DLS) and FTIR measurements. Only one transition can be observed via all detecting methods, revealing the cooperative aggregation of the two distinct temperature-sensitive segments. What is more, the combination of strong hydrophobic interactions among the entire polymer chains and hydrogen bonds of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O…H–N within PNIPAM segments results in the sharp variations at the LCST and gradual variations above the LCST during the phase transition of PNIPAM-b-PVCL aqueous solution upon heating. Additional analysis by perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2Dcos) indicates that the hydrophobic C–H groups have an earlier response than the relatively hydrophilic C
O…H–N within PNIPAM segments results in the sharp variations at the LCST and gradual variations above the LCST during the phase transition of PNIPAM-b-PVCL aqueous solution upon heating. Additional analysis by perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2Dcos) indicates that the hydrophobic C–H groups have an earlier response than the relatively hydrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups during the LCST transition.
O groups during the LCST transition.

 Please wait while we load your content...
Please wait while we load your content...