Tactics for probing aryne reactivity: mechanistic studies of silicon–oxygen bond cleavage during the trapping of (HDDA-generated) benzynes by silyl ethers†
Abstract
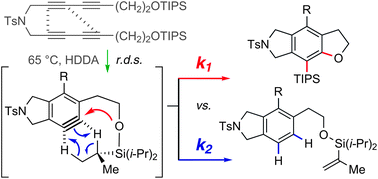
We report mechanistic aspects of the trapping of thermally (HDDA) generated benzyne derivatives by pendant silyl ether groups, which results in net insertion of the pair of benzyne Csp-hydribized carbon atoms into the silicon–oxygen sigma bond. Cross-over experiments using symmetrical, doubly labeled bis-silyl ether substrates established that the reaction is unimolecular in nature. Competition experiments involving either intramolecular or intermolecular dihydrogen transfer clock reactions (from within a TIPS isopropyl group or cyclooctane, respectively) vs. the silyl ether cyclization were used to gain additional insights. We evaluated effects of the steric bulk of the silyl ether trapping group and of the ring-size of the cyclic ether being formed (furan vs. pyran). These types of competition experiments allow the relative rates of various product-determining steps to be determined. This previously has only rarely been possible because aryne formation is typically rate-limiting, making it challenging to probe the kinetics of subsequent trapping reactions. Solvent effects (polarity of the medium) and computational studies were used to probe the question of stepwise vs. concerted pathways for the Si–O insertion.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...