Effects of a disulfide bridge prior to amyloid formation of the ABRI peptide†
Abstract
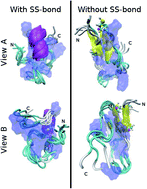
The ABRI peptide is involved in the neurodegenerative disease Familial British Dementia, which has its origin in the misfolding of the peptide. Characterizing the most probable conformations of the monomer in solution can provide insights into how misfolding could occur in the steps prior to aggregation. Specifically, we analyzed the structural effects caused by the formation of a single disulfide bond, which has been reported to be important in amyloid assembly. We used all-atom molecular dynamics simulations with an enhanced sampling technique to obtain the lowest free energy conformations for two cases: the peptide with and without the disulfide bond between residues 5Cys and 22Cys. Bulk measurements on the conformations agree with experiments by elucidating ABRI as a disordered peptide. We find remarkable differences at the microscopic level between the most probable structures; with the disulfide bond the peptide is compact and α-helical, without the bond it is partially extended with slight β-bridges.

 Please wait while we load your content...
Please wait while we load your content...