Self-assembled two-dimensional copper oxide nanosheet bundles as an efficient oxygen evolution reaction (OER) electrocatalyst for water splitting applications†
Abstract
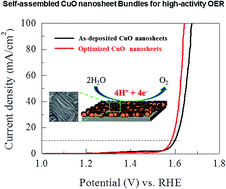
A high activity of a two-dimensional (2D) copper oxide (CuO) electrocatalyst for the oxygen evolution reaction (OER) is presented. The CuO electrode self-assembles on a stainless steel substrate via chemical bath deposition at 80 °C in a mixed solution of CuSO4 and NH4OH, followed by air annealing treatment, and shows a 2D nanosheet bundle-type morphology. The OER performance is studied in a 1 M KOH solution. The OER starts to occur at about 1.48 V versus the RHE (η = 250 mV) with a Tafel slope of 59 mV dec−1 in a 1 M KOH solution. The overpotential (η) of 350 mV at 10 mA cm−2 is among the lowest compared with other copper-based materials. The catalyst can deliver a stable current density of >10 mA cm−2 for more than 10 hours. This superior OER activity is due to its adequately exposed OER-favorable 2D morphology and the optimized electronic properties resulting from the thermal treatment.


 Please wait while we load your content...
Please wait while we load your content...