Three-dimensional conductive networks based on stacked SiO2@graphene frameworks for enhanced gas sensing†
Da
Huang
a,
Zhi
Yang
*ab,
Xiaolin
Li
a,
Liling
Zhang
a,
Jing
Hu
a,
Yanjie
Su
a,
Nantao
Hu
*a,
Guilin
Yin
b,
Dannong
He
b and
Yafei
Zhang
a
aKey Laboratory for Thin Film and Microfabrication of Ministry of Education, Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: zhiyang@sjtu.edu.cn; hunantao@sjtu.edu.cn
bNational Engineering Research Center for Nanotechnology, Shanghai 200241, P. R. China
First published on 3rd October 2016
Abstract
Graphene is an ideal candidate for gas sensing due to its excellent conductivity and large specific surface areas. However, it usually suffers from sheet stacking, which seriously debilitates its sensing performance. Herein, we demonstrate a three-dimensional conductive network based on stacked SiO2@graphene core–shell hybrid frameworks for enhanced gas sensing. SiO2 spheres are uniformly encapsulated by graphene oxide (GO) through an electrostatic self-assembly approach to form SiO2@GO core–shell hybrid frameworks, which are reduced through thermal annealing to establish three-dimensional (3D) conductive sensing networks. The SiO2 supported 3D conductive graphene frameworks reveal superior sensing performance to bare reduced graphene oxide (RGO) films, which can be attributed to their less agglomeration and larger surface area. The response value of the 3D framework based sensor for 50 ppm NH3 and 50 ppm NO2 increased 8 times and 5 times, respectively. Additionally, the sensing performance degradation caused by the stacking of the sensing materials is significantly suppressed because the graphene layers are separated by the SiO2 spheres. The sensing performance decays by 92% for the bare RGO films when the concentration of the sensing material increases 8 times, while there is only a decay of 25% for that of the SiO2@graphene core–shell hybrid frameworks. This work provides an insight into 3D frameworks of hybrid materials for effectively improving gas sensing performance.
Introduction
Chemiresistive sensing devices have been widely studied due to their low consumption and high accuracy.1 Among the various sensing materials that are being explored to fabricate chemiresistive sensors, graphene has recently attracted intense attention because of its room temperature conductivity and large surface area.2,3 The ideal graphene is composed of a single layer of carbon atoms, implying that every carbon atom of graphene can serve as an active site, which not only gives excellent gas adsorption ability, but also makes it very sensitive to adsorbed gas molecules. Thus, various methods have been explored to obtain single layer sheets of graphene. Mechanically-exfoliated graphene was even reported to possess single-molecule sensing properties.4 However, this high sensitivity would also lead to poor selectivity that hinders its practical application, since a variety of gas molecules could be adsorbed on the surface of graphene, which would affect its electronic structure, resulting in changes of electrical resistance.It was reported that better chemical affinity and selectivity can be achieved with functionalized graphene or reduced graphene oxide (RGO).5 RGO, derived from graphene oxide (GO), is usually partially reduced, which makes it a semiconductor material that can alternatively present either p-type or n-type semiconductor behaviors depending on the preparation processes.6,7 Chemically reduced graphene oxide8–10 and thermally reduced graphene oxide (TRGO)11,12 are two kinds of widely investigated RGO. Compared with the graphene prepared by chemical vapor deposition13,14 and mechanical-exfoliation15 and liquid exfoliation16 methods, RGO can be obtained in an easier way, and at larger scales and much lower cost. TRGO is especially controllable and repeatable because the product can only be affected by temperature and time, which will lead to cost-effective, practical implementation of its large-scale fabrication.
Nevertheless, RGO-based gas sensing devices encounter an important challenge in their fabrication process. It is reported that the sensing performance of graphene based materials is seriously influenced by the thickness of their sensing films.17,18 From a theoretical aspect, single layer graphene without any stacking possesses the largest specific area and best sensing performance. However, it is very difficult to obtain a uniform ultrathin RGO coating on the electrodes because whether through thermal annealing or chemical reduction, overlapping and agglomeration are inevitable,10,12,19 which can be attributed to the unavoidable agglomeration of nanomaterials during drying processes,20 as well as the well-known coffee ring effect.21,22 The thickness of sensing films is a pivotal factor that impacts the sensing performance of chemiresistive gas sensors. Besides, even if a single layer RGO-based sensing device can be fabricated, its stability is still a problem because the single layer RGO can be easily affected by other external forces in addition to the concentrations of target gases.
In order to solve these problems, herein, effectively enhanced gas sensitive 3D RGO frameworks were fabricated through a self-assembly method using SiO2 spheres as supporting substrates. SiO2 spheres were employed due to their ease of preparation, smooth surfaces, controllable sizes, and uniform morphologies. In addition, the interspace between the stacked spheres would offer channels for gas flow. Owing to their negatively charged nature, GO nanosheets can be easily adsorbed on the positively charged surface of modified SiO2 spheres.23 The resulting SiO2@RGO core–shell composites with ultrathin graphene shell cladding on the surfaces of SiO2 spheres were expected to solve the above-mentioned problems since this unique structure can (1) form 3D network structure with valid contact, (2) avoid the agglomeration and stacking of graphene, (3) provide larger surface area for the adsorption of gas molecules, (4) maintain the stable electrical conductivity of the whole electrode. In order to confirm the performance improvement resulting from the 3D frameworks, gas sensing measurements were carried out using a homemade gas-control system. The results meet our expectation with a higher response value and excellent stability. As a consequence, the sensing performance of SiO2@RGO-based sensors was significantly improved compared with bare RGO-based sensors, indicating that building 3D conductive networks is an efficient method for gas sensing applications. Moreover, the sphere supported construction provides an effective approach to suppress the effect of the thickness of sensing films, which makes the gas sensing device fabricated through the dip-coating method more controllable.
Experimental
Preparation of SiO2 spheres
SiO2 spheres were prepared by a reported method based on the hydrolysis and condensation of tetraethylorthosilicate (TEOS).24 A mixture of 10 mL of methanol and 30 mL of n-butanol was used as the solvent for the reaction, which was stirred in a flask. Then 3 mL of ammonium hydroxide (27–30 wt% NH3) was added into the flask and stirred for 10 min. After that, 1.5 mL of TEOS was added drop by drop. The liquid mixture were kept stirring for another 2 hours to form the SiO2 spheres, which were centrifuged and washed repeatedly with ethanol and water to remove the remaining solvent and extra TEOS, and finally kept in ethanol.Surface modification of SiO2 spheres
In order to modify the SiO2 spheres with amino groups, 100 μL of 3-aminopropyl trimethoxysilane (APTMS, 97%) was added in the suspension of SiO2 spheres and stirred overnight. The APTMS-modified SiO2 spheres were centrifuged and washed repeatedly with ethanol and water to remove the extra APTMS and finally kept in ethanol for further use.Preparation of GO
The GO used here was synthesized through a modified Hummer's method mentioned in our previous reports.25,26Preparation of SiO2@GO composites
200 μL of SiO2 sphere suspensions (5 mg mL−1) were added into 5 mL of stirring GO suspension (0.02 mg mL−1) drop by drop. The mixture was kept stirring for 12 h. After that, the suspension was centrifuged to remove the extra GO and re-dispersed in water for further use.Fabrication of SiO2@TRGO composite-based sensing devices
The interdigital electrodes used in the experiment were fabricated by reported standard microfabrication procedures.25 Firstly, 20 nm Ti and 180 nm Au were sputtered on to a patterned photoresist mold in sequence. After that, the photoresist was removed with acetone. The as-obtained electrodes were cleaned by sonication and washed with ethanol and deionized water, and finally dried by nitrogen flow. A typical protocol for the fabrication of a SiO2@TRGO composite-based sensing device was designed as follows: 2 μL of SiO2@GO suspension was deposited on the electrode gap using a micro syringe. After evaporating it naturally, the 3D SiO2@GO frameworks can be formed. Then the devices were put in a vacuum oven at 80 °C for 1 h to evaporate the extra water. This low temperature heating process will also benefit the contact between the sensing materials and the electrodes. After that, the electrodes with SiO2@GO composite deposits were kept in an oven at 200 °C for 90 min.Fabrication of bare TRGO-based sensing devices
Sensing devices based on bare TRGO were also fabricated according to the same procedure except that the SiO2@GO composites were replaced by bare GO.Characterization
The morphologies of the samples were observed by using a field emission scanning electron microscope (FE-SEM, Ultra Plus, Carl Zeiss, Germany) and transmission electron microscope (TEM, JEM-2100, JEOL, Japan). Atomic force microscopy was performed using a dimension icon instrument (AFM, Veeco, USA). Thermogravimetric analyses of the samples were carried out with a thermogravimetric analyzer (TGA, Pyris 1, Perkin Elmer, USA) at a heating rate of 5 °C min−1 from 50 to 700 °C in flowing air. Fourier transform infrared (FT-IR) spectra were recorded on a spectrometer (VERTEC 70, Bruker, Germany) with the range of 4000–400 cm−1, using KBr as bases. Raman spectroscopy was characterized using a Raman microscope (Raman, DXRTM, Thermo Fisher, America) with a laser wavelength at 532 nm. X-ray photoelectron spectra were acquired using a spectrometer (XPS, Kratos Axis Ultra DLD, Japan) with a monochromatic Al Kα source (1486.6 eV).Surface area measurement
Cyclic voltammetry (CV) measurement was performed with an electrochemical workstation (CHI760E, Chenhua, Shanghai, China) at room temperature, using a conventional three-electrode system consisting of an ITO electrode (0.5 cm × 0.5 cm), a platinum gauze electrode, and a saturated calomel electrode as the working electrode, the counter electrode, and the reference electrode, respectively. The surface areas were also determined by BET measurement (Micromeritics ASAP 2020 M, USA).Gas sensing measurements
Gas sensing measurements were carried out using a homemade gas-control system as illustrated in our previous reports. There are two gas paths that can flow through the test chamber, which are named background gas and target gas, respectively. The background gas is dry compressed air, while the target gas consists of analytes and dry compressed air.25 The concentrations of analytes can be controlled by mass flow controllers (MFC, Beijing Qixing Co., Ltd, China). The background gas and target gas can be controlled to flow through the test chamber alternatively by two-way valves. A common test process taking NH3 for example is demonstrated as follows. Firstly, a sensor is put into the test chamber and dry compressed air is purged into the test chamber as background gas and into the mixing chamber as diluent gas. At the same time, NH3 gas is also imported into the mixing chamber. The conductance of the sensor and the NH3 concentration of the target gas both reach a stable status soon. After that, the target gas is controlled to flow through the test chamber instead of background gas. After a single response process, the sensor recovers in the background gas again with the aid of an IR lamp that can stimulate the desorption process on the device. When the conductance of the sensor recovers to its initial value, NH3 with different concentrations can be switched into the chamber for another new response.All gas sensing tests were carried out in the same environment at room temperature (25 °C) and a certain relative humidity (50%), which was controlled by an air conditioner. The speed of gas flowing through the test chamber was controlled to be 1 L min−1. The response of the gas sensor was evaluated by the change of the electrical conductance of the gas sensor, which was measured by a precision semiconductor parameter analyzer (Agilent 4156C) with a working voltage of 500 mV.
The response value (R) is calculated according to the following equation:
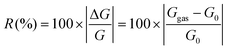 | (1) |
Results and discussion
Preparation and characterization of SiO2@TRGO composites
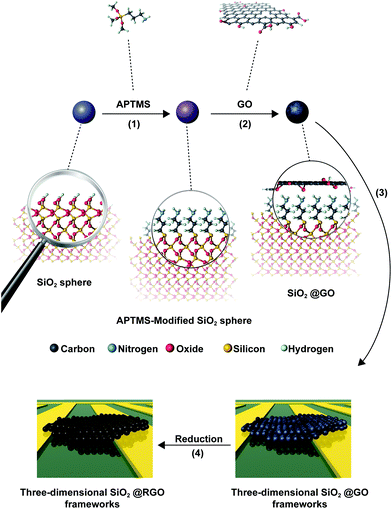
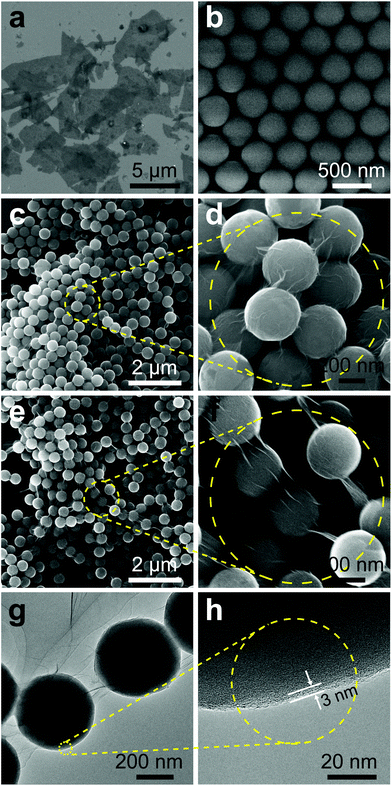
An electrostatic self-assembly approach was used to prepare SiO2@TRGO composites, as shown in Fig. 1. The cladding of GO on the SiO2 spheres was driven by the electrostatic interactions between the two species. Firstly, SiO2 spheres were modified with APTMS to graft –NH2 on their surfaces, rendering the oxide surface positively charged. There were a large number of hydroxyl and carboxylic groups on the surface of the GO nanosheets, making it very easy for them to be attached on the APTMS-modified SiO2 spheres. When positively charged APTMS-modified SiO2 spheres were mixed with negatively charged GO, a GO-encapsulated structure would be quickly formed. In order to avoid the aggregation of RGO in the reduction process, the materials were deposited on the electrodes to form 3D SiO2@GO frameworks for the following in situ reduction process. The as-obtained SiO2@GO frameworks were then reduced to a SiO2@TRGO conductive network through thermal annealing at 200 °C for 90 min. This temperature was determined according to previous reports.27,28The particle size of the GO obtained here was estimated to be approximately 1–5 μm from the SEM image shown in Fig. 2a. The TEM and AFM results shown in Fig. S1† reveal the ultrathin feature of as-synthesized GO, with a thickness of 0.74 nm. From the SEM image in Fig. 2b, we can clearly observe that as-obtained SiO2 spheres possess smooth surfaces and uniform sizes with approximately 430 nm diameter, which can adapt to the size of the GO. The morphology of SiO2@GO is presented in Fig. 2c. It can be found that the SiO2 spheres were arranged side by side after the cladding of the GO, ensuring close contact with each sphere in the whole framework. The SiO2@GO composites showed a crinkled and rough texture due to the flexible nature of GO nanosheets, which can be seen more clearly in the magnified view shown in Fig. 2d, indicating the presence of ultrathin GO layer cladding. It is noted that the suitable sizes of GO and SiO2 spheres, and the modification of SiO2 by APTMS are very important for the formation of SiO2@GO core–shell structures, without which failure would result, as shown in Fig. S2.† It can be seen in Fig. S2a† that several smaller SiO2 spheres (110 nm) are encapsulated by single GO sheets. If the SiO2 spheres are directly added into the GO suspension without the modification by APTMS, the GO nanosheet shell will even not be formed, as shown in Fig. S2b.† By comparing Fig. 2e and f, the morphology of SiO2@TRGO composites exhibited little variation after reduction except for the tenser graphene junction caused by the thermal annealing process. The tighter contact will be beneficial for the connectivity of the whole conductive network.
TEM analysis was carried out to verify the hetero-structures of as-prepared SiO2@GO core–shell composites as shown in Fig. 2g and h. The slightly curved conjunctive GO between adjacent SiO2 spheres can be clearly observed in Fig. 2g, which is consistent with the SEM results. The wrinkled GO nanosheets tightly covering the surfaces of the SiO2 spheres was not obviously seen in the TEM images due to the low electron contrast. However, this can be confirmed by the lattice differences in the wrinkled GO covering on the surface of a typical SiO2 sphere, which is displayed in a magnified view shown in Fig. 2h, confirming the ultrathin thickness of the graphene shell (less than 3 nm) cladding the surface of the SiO2 spheres. The SEM and TEM results proved that the SiO2 spheres were uniformly encapsulated by the GO nanosheets and the SiO2@GO-based 3D frameworks were successfully formed. After being reduced from SiO2@GO to SiO2@TRGO, the electrical conductivity of the 3D frameworks was significantly enhanced. This can be proved by the IV curve presented in Fig. S3,† suggesting that the GO shells covering the SiO2 were successfully reduced without agglomeration.
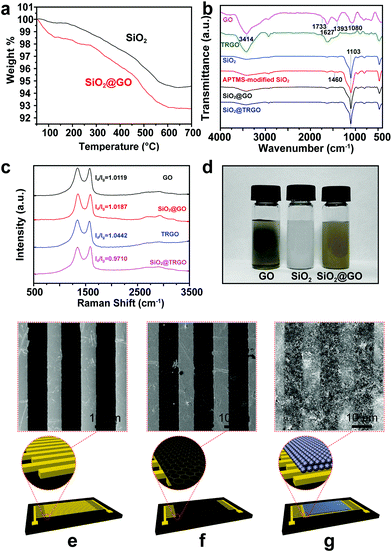
In order to measure the GO content in the SiO2@GO composites, TGA analysis was carried out. As shown in Fig. 3a, the black and red curves correspond to the TGA results of bare SiO2 and SiO2@GO composites, respectively. Compared with bare SiO2, the SiO2@GO composites began to lose weight even under 100 °C due to the thermal instability of GO,10 and eventually remained at 92.8% of the initial weight, which is lower than that of bare SiO2 (94.5%), implying a very low mass fraction (less than 2%) of GO in the composites.
FT-IR spectra were collected to confirm the successful reduction of GO and the self-assembly process of SiO2@GO. A typical spectrum of GO is shown in Fig. 3b at the top. The broad, intense peaks located at 3414 and 1393 cm−1 can be attributed to O–H stretching and bending vibration, respectively.29 The presence of C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can be confirmed by the peaks appearing at 1080, 1627 and 1733 cm−1, respectively, suggesting that the oxidation of graphite and the formation of GO were successfully achieved.30,31 The FT-IR spectrum of the low-temperature thermally reduced product confirmed the successful reduction of GO. The absorption intensities at 1080 and 1393 cm−1 decreased dramatically due to the significant decrease of C–O and O–H. The weakened adsorption peaks at 3414 cm−1 and the fingerprint region below 1000 cm−1 also revealed the reduced –OH and other oxygen groups, indicating the reduction of GO. For the SiO2 spheres, the characteristic peak at 1103 cm−1 appears due to the Si–O stretching vibration. After the modification with APTMS, the N–H stretching vibration band at 1460 cm−1 appears.32 The FT-IR spectra of SiO2@GO and SiO2@TRGO are similar to APTMS-modified SiO2 because the SiO2@GO contains less than 2% GO. The peaks of GO are significantly covered by the peaks of SiO2. However, it can be observed that the assembling process of SiO2 and GO leads to the appearance of some peaks in the region between 530 and 730 cm−1, which decrease in that of SiO2@TRGO, coinciding with the change from GO to TRGO caused by the reduction process.
O can be confirmed by the peaks appearing at 1080, 1627 and 1733 cm−1, respectively, suggesting that the oxidation of graphite and the formation of GO were successfully achieved.30,31 The FT-IR spectrum of the low-temperature thermally reduced product confirmed the successful reduction of GO. The absorption intensities at 1080 and 1393 cm−1 decreased dramatically due to the significant decrease of C–O and O–H. The weakened adsorption peaks at 3414 cm−1 and the fingerprint region below 1000 cm−1 also revealed the reduced –OH and other oxygen groups, indicating the reduction of GO. For the SiO2 spheres, the characteristic peak at 1103 cm−1 appears due to the Si–O stretching vibration. After the modification with APTMS, the N–H stretching vibration band at 1460 cm−1 appears.32 The FT-IR spectra of SiO2@GO and SiO2@TRGO are similar to APTMS-modified SiO2 because the SiO2@GO contains less than 2% GO. The peaks of GO are significantly covered by the peaks of SiO2. However, it can be observed that the assembling process of SiO2 and GO leads to the appearance of some peaks in the region between 530 and 730 cm−1, which decrease in that of SiO2@TRGO, coinciding with the change from GO to TRGO caused by the reduction process.
Raman spectroscopy, a powerful tool for the identification of graphene and its derivatives, was also employed for the further analysis of GO, TRGO, SiO2@GO and SiO2@TRGO, as shown in Fig. 3c. Two typical features of the D band and G band of graphene can be prominently observed for all samples. The D band located at 1343 cm−1 arises from the breathing mode of k-point phonons of A1g symmetry, and the G band around 1592 cm−1 is assigned to the first-order scattering of E2g photon of sp2 atoms.33 The ID/IG of TRGO (ID/IG = 1.0442) is also higher than that of GO (ID/IG = 1.0119) due to the decrease in the average size of the sp2 domains but increase in numbers, which is inconsistent with the previous report.34 However, the ID/IG ratio of SiO2@TRGO (ID/IG = 0.9710) is lower than that of SiO2@GO (ID/IG = 1.0187). This can be mainly attributed to the supporting function of the SiO2 spheres, which makes SiO2@GO more uniformly heated, thus hindering the generation of defects during the reduction process with the restoration of sp2 bonds in GO nanosheets. As a result, the 3D RGO network built on SiO2 spheres with higher surface areas and larger average sizes of the sp2 domains was successfully obtained.
After being encapsulated by GO, the color of the SiO2 aqueous suspension changed from white to brown, which can be visually confirmed (Fig. 3d). It can also be found that the as-synthesized SiO2@GO composites maintained excellent dispersion behaviors, which is important for uniform coating of the sensing materials on the electrodes. The schematic structures and corresponding SEM images of the bare interdigital electrodes, the 2D TRGO film deposited electrodes, and the 3D SiO2@TRGO frameworks deposited electrodes are illustrated in Fig. 3e–g. There are little visual differences between the 2D TRGO films deposited electrodes and the bare interdigital electrodes due to the transparent nature of ultrathin graphene films. In contrast, it can be clearly observed that the 3D SiO2@TRGO frameworks uniformly cover the electrodes, indicating the uniform coating of 3D conductive sensing networks on the electrodes.
X-ray photoelectron spectroscopy (XPS) was also employed to investigate the elemental compositions and chemical states of SiO2@GO, SiO2@TRGO, as shown in Fig. 4. The C 1s, O 1s, Si 2s and Si 2p peaks can be distinctly observed in the survey scan curves of both SiO2@GO (Fig. 4a) and SiO2@TRGO (Fig. 4b). There is a higher C content and lower O content in the SiO2@TRGO than those in SiO2@GO, which is caused by the thermal reduction. There is also a small peak of N 1s observed in both spectra, which may be due to the APTMS left on the surface of SiO2 spheres. The successful reduction of SiO2@GO can be proved by the C 1s XPS spectra of SiO2@GO and SiO2@TRGO shown in Fig. 4c and d. Compared with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks, the peaks of the oxygen-containing groups marginally decreased after thermal reduction, indicating the successful reduction of GO on the surface of the SiO2.
C peaks, the peaks of the oxygen-containing groups marginally decreased after thermal reduction, indicating the successful reduction of GO on the surface of the SiO2.
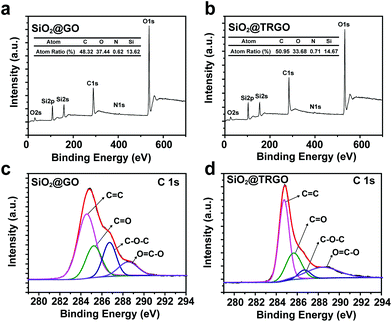 | ||
| Fig. 4 XPS survey scan spectra of (a) SiO2@GO and (b) SiO2@TRGO. De-convoluted C 1s XPS spectra of (c) SiO2@GO and (d) SiO2@TRGO. | ||
Gas sensing performance of SiO2@TRGO composites
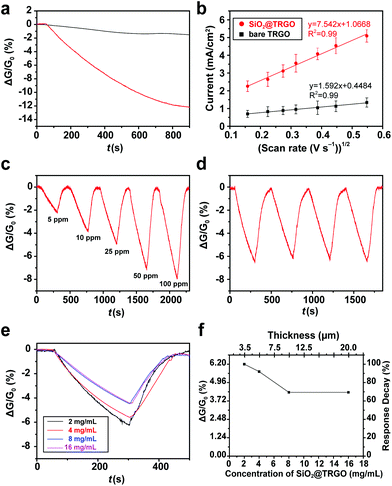
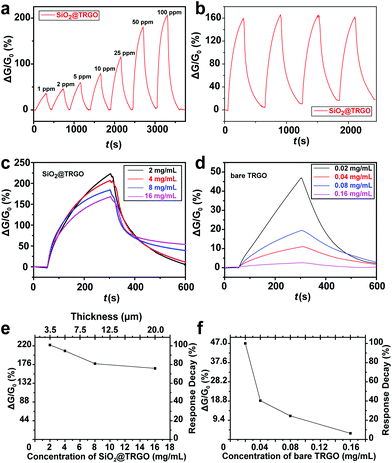
The sensing performance of the SiO2@TRGO composites was firstly evaluated by monitoring their response to ammonia (NH3), which is a common toxic gas used in various fields, such as industrial and medical processes.35–37 RGO-based NH3 gas sensors were widely and frequently studied before, however, with unsatisfactory performances.27,28,38 The common methods used to improve the sensing performance of RGO-based sensors for NH3 are to combine RGO with other semiconductor materials like metal oxide39–42 or conducting polymers.43–45 This work focuses on the study of structure rather than materials. The as-synthesized 3D SiO2@TRGO frameworks were expected to enhance the sensing performance of RGO-based NH3 sensors with their optimized structures.A single response curve of SiO2@TRGO for 50 ppm NH3 is shown in Fig. 5a. It can be clearly observed that the conductance of the devices was stable in the air background for the first 50 s. When the NH3 target gas was introduced into the chamber, the conductance of the SiO2@TRGO-based device significantly decreased, which corresponds to the properties of p-type semiconductors.46 In this sensing process, the charge of the adsorbed electron-donated NH3 is transferred to the SiO2@TRGO network, reducing the concentration of hole carriers in p-type TRGO, leading to a decreased conductivity of the devices.38,47 A response value as high as 12.1% can be reached after a period of 850 s. For the purpose of comparison, the response curve for 50 ppm NH3 of bare TRGO is shown in Fig. 5a as well. The response value of the bare TRGO device is only about 1.5%, much lower than that of SiO2@TRGO. Since SiO2 is an insulator, the 8 times enhancement is mainly attributed to the 3D networks of SiO2@TRGO, which offer a large surface area and more active sites for the adsorption of NH3 molecules. Considering that a significant decrease of the conductance of the sensors can be observed during the preliminary detection period, an effective response time of 250 s was defined for the following tests.
The significantly improved performance of the SiO2@TRGO-based sensor can be explained as follows. The SiO2 spheres effectively separate the graphene layers and hinder agglomeration, making full use of the large surface area of graphene nanosheets, as shown in Fig. S4.† Compared with the 2D TRGO sensing films, the surface area of the 3D SiO2@TRGO networks is increased by factors of π based on the following equation,
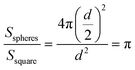 | (2) |
CV tests were carried out in a solution of 0.1 M KCl and 10 mM K3Fe(CN)6 to confirm the increased surface area resulting from the 3D SiO2@TRGO frameworks (Fig. S6†). 200 μg of bare TRGO and 200 μg of SiO2@TRGO were uniformly coated on a 0.5 cm × 0.5 cm indium-tin-oxide (ITO) anode respectively for the CV tests. The effective surface area can be calculated from the Randles–Sevcik equation:
| ip = (2.687 × 105)n3/2v1/2D1/2AC | (3) |
In order to study the sensing performance of the devices for different concentrations of NH3, the flowmeter was regulated to introduce NH3 gas from 5 to 100 ppm into the test chamber in turn. As shown in Fig. 5c, the response values of SiO2@TRGO for 5, 10, 25, 50 and 100 ppm of NH3 in 250 s are 2.2%, 3.7%, 4.9%, 6.5% and 7.9%, respectively. The response value can be fully recovered to its initial value with the assistance of an IR-lamp.
Furthermore, the response of the SiO2@TRGO device also presents excellent reversibility, as shown in Fig. 5d. A reversibility test of the SiO2@TRGO device with NH3 over 4 cycles was carried out by exposing the device to 50 ppm of NH3 repeatedly. The stability of the device can be proved by the fact that the response value still remained at the same level after several cycles of exposure to NH3 and recovery in air. Its excellent stability suggests that the SiO2@TRGO 3D conductive network-based device can be repeatedly used, exhibiting great potential in practical uses.
The thickness of sensing films is an important factor in gas sensing.48–51 The sensing performance of RGO based sensors has also been reported to be seriously affected by their thickness.17,18 It is reported that the response value of a chemically modified graphene-based sensor decreased more than 2 times when the thickness of the sensing film was increased from 10 nm to 50 nm.17 Generally, the thickness of the sensing film on the electrodes should be controlled to be as thin as possible to achieve better sensitivity. However, ultrathin sensing films are much harder for large scale production and practical uses because of their worse stability and the difficulty in controlling them. Moreover, the increase in sample concentration tends to generate much heavier agglomeration. The 3D networks of RGO can solve this problem to some extent. To measure the effect of the thickness of samples on the sensors, sensing devices with different concentration/thickness dropcast onto the electrodes were prepared for testing. The IV curves (Fig. S3†) indicate that the electrical conductivity was enhanced with the increase in concentration/thickness. However, the sensing performance was slightly decreased with the increase in concentration/thickness, as shown in Fig. 5e. The response values of SiO2@TRGO-based devices were measured as a function of the concentration of samples, as shown in Fig. 5f. It can be found that the response value only decayed by 30% and became stable when the concentration was increased 8 times. Meanwhile, the thickness of the SiO2@TRGO composites on the electrodes was increased from 3.5 to 20 μm, which was measured by SEM as shown in Fig. S6.† A high performance is still maintained even with a thickness of 20 μm, revealing that the stacking of SiO2@TRGO did not make much difference to the performance of the SiO2@TRGO sensor.
Since the response of bare TRGO based sensing devices towards NH3 is not so obvious, a gas sensing test for nitrogen dioxide (NO2) was also performed as contrast experiment to further demonstrate the proof-of-concept. In contrast to NH3, NO2 leads to an increase in the electrical conductance of p-type TRGO due to its strong electron acceptor properties. The hole carriers in TRGO are accumulated in the charge transfer process from TRGO to NO2.52 The response towards NO2 is usually stronger than NH3 at the same concentration level. As shown in Fig. 6a, the SiO2@TRGO based sensor shows a response of 35.5% towards 1 ppm NO2 in 250 s, and the response value increased to 206.1% for 100 ppm NO2. A UV-lamp was used here to help the devices reach full recovery.
A reversibility test for 50 ppm NO2 for the SiO2@TRGO device for NH3 over 4 cycles was also performed, as shown in Fig. 6b. The repeatable results indicate that the SiO2@TRGO 3D structure has great reversibility for both electron-donor-type gas molecules and electron-acceptor-type gas molecules. The effect of the thickness of sensing materials is also studied here by performing a contrast experiment between the SiO2@TRGO based devices and the bare TRGO based devices.
Similar to the NH3 cases, it can be observed from the response curves for 50 ppm NO2 shown in Fig. 6c that devices with higher concentration/thickness sensing materials have poorer sensing performances and slower recovery rates. This is due to the inability of UV-light to penetrate the sensing films effectively when the sensing film is too thick. Compared with the response curves of bare TRGO based sensing devices shown in Fig. 6d, the effect of concentration/thickness on SiO2@TRGO is effectively restrained. The decrease trends of the response values of the SiO2@TRGO based devices and bare TRGO based devices are shown in Fig. 6e and f, respectively. For the SiO2@TRGO based devices, the response value decreased by only 25% when the concentration increased 8 times. In contrast, for the bare TRGO based devices, the concentration increased 7 times with a significant response decay of 92%. Usually, the sensing performance of devices fabricated through the dip-coating method is not very stable, with their response value being different from batch to batch. For the SiO2@TRGO-based gas sensor, the thickness of the sensing film is not only more controllable and easier to measure, but also induces less influence on the performance of sensing devices, which will significantly facilitate the practical commercial application of gas sensing devices fabricated through the dip-coating method. Briefly, better and more stable performance can be achieved through 3D conductive networks based on stacked SiO2@graphene in gas sensing.
The influence of humidity on the sensing devices was also investigated and is demonstrated in Fig. S7.† It can be concluded that the conductance of SiO2@TRGO will increase with increasing relative humidity (RH). The conductance decreases by about 11% when it is transferred from wet air (RH = 76.8%) to dry air (RH = 0.0%). Note that the conductance tends to be stable in the range of 30%–80%, indicating that the SiO2@TRGO based sensor is stable in most situations.
Working temperature is also considered to be an important factor that affects the sensing performance of sensors. In order to study the influence of temperature, an IR lamp was used to heat the sensing chamber at different distances to simulate different temperatures. Sensing tests for NO2 were carried out at 25 °C, 50 °C and 75 °C, respectively. It can be concluded from the results in Fig. S8a† that the conductance of the SiO2@TRGO sensor increases with the increasing temperature. However, the response curves shown in Fig. S8b† demonstrate that the highest response value is achieved at 25 °C. Though the response value becomes lower when under high temperature conditions, the response time and recovery time are greatly shortened with the increase in temperature, which is very useful in its practical applications.
Fig. S9† presents the selectivity of SiO2@TRGO. The response value of the SiO2@TRGO for 50 ppm NO2 is as high as 162.5% while the value is only 6.2% for 50 ppm NH3. The fact that the SiO2@TRGO based sensors are more sensitive towards NO2 than NH3 corresponds well with the previously reported work on graphene based sensing devices that were prepared with thermal treatment.28,53,54 However, it still shows relatively good selectivity when compared with the 1% saturated concentration vapors of some other VOC vapors, such as chloroform, dichloromethane, toluene, formaldehyde and methanol.
Some other room-temperature NH3 and NO2 gas sensors based on graphene that were previously reported are shown in Table S1.†17,18,28,53–57 Even though the sensing performances of the SiO2@TRGO framework based sensors presented here are relatively comparable, further studies are needed, including optimization of both the functional graphene and supporting materials. For example, chemically modified graphene on different semiconductor spheres is expected to be investigated in follow-up work.
Conclusions
In conclusion, we have demonstrated 3D conductive networks based on SiO2@TRGO composites for enhanced gas sensing performance at room temperature. A facile, simple, quick, convenient, and low cost electrostatic self-assembly method was used here to prepare SiO2@GO composites which can be reduced to SiO2@TRGO by thermal annealing. The sensing performance of 3D conductive network-based sensors can be less affected by the thickness of the sensing film, which is a great breakthrough in devices fabricated by dip-coating methods. The 3D conductive networks constructed by these simple and low-cost core–shell composites are expected to be used for detecting different types of gases in practical applications.Acknowledgements
The authors gratefully acknowledge financial support by the National Key Research and Development Program of China (2016YFC0102700), the National Natural Science Foundation of China (61671299 and 51402190), Shanghai Science and Technology Grant (16JC1402000 and 13ZR1429400), the Program of Shanghai Academic/Technology Research Leader (15XD1525200), Shanghai Jiao Tong University Agri-X Funding (Agri-X2015007), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. We also acknowledge analysis support from the Instrumental Analysis Center of Shanghai Jiao Tong University and the Center for Advanced Electronic Materials and Devices of Shanghai Jiao Tong University.Notes and references
- T. Wang, Y. L. Guo, P. B. Wan, H. Zhang, X. D. Chen and X. M. Sun, Small, 2016, 12, 3748–3756 CrossRef CAS PubMed.
- W. J. Yuan and G. Q. Shi, J. Mater. Chem. A, 2013, 1, 10078–10091 CAS.
- T. Wang, D. Huang, Z. Yang, S. S. Xu, G. L. He, X. L. Li, N. T. Hu, G. L. Yin, D. N. He and L. Y. Zhang, Nano-Micro Lett., 2016, 8, 95–119 CrossRef.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- S. Basu and P. Bhattacharyya, Sens. Actuators, B, 2012, 173, 1–21 CrossRef CAS.
- X. S. Wu, M. Sprinkle, X. B. Li, F. Ming, C. Berger and W. A. de Heer, Phys. Rev. Lett., 2008, 101, 026801 CrossRef PubMed.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS PubMed.
- J. L. Zhang, H. J. Yang, G. X. Shen, P. Cheng, J. Y. Zhang and S. W. Guo, Chem. Commun., 2010, 46, 1112–1114 RSC.
- M. H. Xu, J. Chai, N. T. Hu, D. Huang, Y. X. Wang, X. L. Huang, H. Wei, Z. Yang and Y. F. Zhang, Nanotechnology, 2014, 25, 395602 CrossRef PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- H. A. Becerril, J. Mao, Z. F. Liu, R. M. Stoltenberg, Z. N. Bao and Y. S. Chen, ACS Nano, 2008, 2, 463–470 CrossRef CAS PubMed.
- M. Acik, G. Lee, C. Mattevi, M. Chhowalla, K. Cho and Y. J. Chabal, Nat. Mater., 2010, 9, 840–845 CrossRef CAS PubMed.
- A. Reina, X. T. Jia, J. Ho, D. Nezich, H. B. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed.
- K. Yan, L. Fu, H. L. Peng and Z. F. Liu, Acc. Chem. Res., 2013, 46, 2263–2274 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Y. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- W. J. Yuan, A. R. Liu, L. Huang, C. Li and G. Q. Shi, Adv. Mater., 2013, 25, 766–771 CrossRef CAS PubMed.
- T. Quang Trung, H. Huynh Tran My, D.-H. Yoo, C. Tran Viet, S. H. Hur, J. S. Chung, E. J. Kim and P. A. Kohl, Sens. Actuators, B, 2014, 194, 45–50 CrossRef.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- S. Mao, Z. H. Wen, S. Q. Ci, X. R. Guo, K. Ostrikov and J. H. Chen, Small, 2015, 11, 414–419 CrossRef CAS PubMed.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827–829 CrossRef CAS.
- H. Hu and R. G. Larson, J. Phys. Chem. B, 2002, 106, 1334–1344 CrossRef CAS.
- Y. Y. Wang, L. L. Zhang, N. T. Hu, Y. Wang, Y. F. Zhang, Z. H. Zhou, Y. H. Liu, S. Shen and C. S. Peng, Nanoscale Res. Lett., 2014, 9, 1–12 CrossRef PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- N. T. Hu, Y. Y. Wang, J. Chai, R. G. Gao, Z. Yang, E. S. W. Kong and Y. F. Zhang, Sens. Actuators, B, 2012, 163, 107–114 CrossRef CAS.
- N. T. Hu, L. Meng, R. G. Gao, Y. Y. Wang, J. Chai, Z. Yang, E. S. W. Kong and Y. F. Zhang, Nano-Micro Lett., 2011, 3, 215–222 CrossRef CAS.
- G. H. Lu, L. E. Ocola and J. H. Chen, Appl. Phys. Lett., 2009, 94, 083111 CrossRef.
- G. H. Lu, L. E. Ocola and J. H. Chen, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994–1998 CrossRef CAS.
- D. Li, M. B. Mueller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- W. F. Chen and L. F. Yan, Nanoscale, 2010, 2, 559–563 RSC.
- D. M. Gao, Z. P. Zhang, M. H. Wu, C. G. Xie, G. J. Guan and D. P. Wang, J. Am. Chem. Soc., 2007, 129, 7859–7866 CrossRef CAS PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, K. A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577–2583 CrossRef CAS.
- B. Timmer, W. Olthuis and A. van den Berg, Sens. Actuators, B, 2005, 107, 666–677 CrossRef CAS.
- S. L. Bai, C. Z. Sun, P. B. Wan, C. Wang, R. X. Luo, Y. P. Li, J. F. Liu and X. M. Sun, Small, 2015, 11, 306–310 CrossRef CAS PubMed.
- P. B. Wan, X. M. Wen, C. Z. Sun, B. K. Chandran, H. Zhang, X. M. Sun and X. D. Chen, Small, 2015, 11, 5409–5415 CrossRef CAS PubMed.
- G. H. Lu, S. Park, K. Yu, R. S. Ruoff, L. E. Ocola, D. Rosenmann and J. H. Chen, ACS Nano, 2011, 5, 1154–1164 CrossRef CAS PubMed.
- Q. Feng, X. Li, J. Wang and A. M. Gaskov, Sens. Actuators, B, 2016, 222, 864–870 CrossRef CAS.
- S. Mao, S. M. Cui, G. H. Lu, K. H. Yu, Z. H. Wen and J. H. Chen, J. Mater. Chem., 2012, 22, 11009–11013 RSC.
- G. Singh, A. Choudhary, D. Haranath, A. G. Joshi, N. Singh, S. Singh and R. Pasricha, Carbon, 2012, 50, 385–394 CrossRef CAS.
- Z. Sun, D. Huang, Z. Yang, X. L. Li, N. T. Hu, C. Yang, H. Wei, G. L. Yin, D. N. He and Y. F. Zhang, IEEE Electron Device Lett., 2015, 36, 1376–1379 CrossRef CAS.
- X. Huang, N. Hu, R. Gao, Y. Yu, Y. Wang, Z. Yang, E. S.-W. Kong, H. Wei and Y. Zhang, J. Mater. Chem., 2012, 22, 22488–22495 RSC.
- N. T. Hu, Z. Yang, Y. Y. Wang, L. L. Zhang, Y. Wang, X. L. Huang, H. Wei, L. M. Wei and Y. F. Zhang, Nanotechnology, 2014, 25, 025502 CrossRef PubMed.
- Y. L. Guo, T. Wang, F. H. Chen, X. M. Sun, X. F. Li, Z. Z. Yu, P. B. Wan and X. D. Chen, Nanoscale, 2016, 8, 12073–12080 RSC.
- J. Moser, A. Verdaguer, D. Jimenez, A. Barreiro and A. Bachtold, Appl. Phys. Lett., 2008, 92, 123507 CrossRef.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301–306 CrossRef CAS PubMed.
- S. M. Cui, H. H. Pu, S. A. Wells, Z. H. Wen, S. Mao, J. B. Chang, M. C. Hersam and J. H. Chen, Nat. Commun., 2015, 6, 8632 CrossRef CAS PubMed.
- J. F. Chang, H. H. Kuo, I. C. Leu and M. H. Hon, Sens. Actuators, B, 2002, 84, 258–264 CrossRef CAS.
- G. Sakai, N. Matsunaga, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2001, 80, 125–131 CrossRef CAS.
- S. L. Bai, C. Z. Sun, H. Yan, X. M. Sun, H. Zhang, L. Luo, X. D. Lei, P. B. Wan and X. D. Chen, Small, 2015, 11, 5807–5813 CrossRef CAS PubMed.
- B. Cho, J. Yoon, M. G. Hahm, D. H. Kim, A. R. Kim, Y. H. Kahng, S. W. Park, Y. J. Lee, S. G. Park, J. D. Kwon, C. S. Kim, M. Song, Y. Jeong, K. S. Nam and H. C. Ko, J. Mater. Chem. C, 2014, 2, 5280–5285 RSC.
- F. Yavari, Z. P. Chen, A. V. Thomas, W. C. Ren, H.-M. Cheng and N. Koratkar, Sci. Rep., 2011, 1, 166 Search PubMed.
- K. Yu, P. Wang, G. Lu, K.-H. Chen, Z. Bo and J. Chen, J. Phys. Chem. Lett., 2011, 2, 537–542 CrossRef CAS.
- R. Ghosh, A. Midya, S. Santra, S. K. Ray and P. K. Guha, ACS Appl. Mater. Interfaces, 2013, 5, 7599–7603 CAS.
- R. K. Paul, S. Badhulika, N. M. Saucedo and A. Mulchandani, Anal. Chem., 2012, 84, 8171–8178 CrossRef CAS PubMed.
- D. Le Thai, D.-J. Kim, T. Tran Quang, D. Vinh Quang, B.-Y. Kim, H. K. Moon and N.-E. Lee, Adv. Funct. Mater., 2015, 25, 883–890 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental setup of gas sensing test, more TEM, SEM and AFM images, IV curves, schematic illustration of the increased surface area, cyclic voltammograms, and a table for comparison of sensing performance. See DOI: 10.1039/c6nr06465e |
| This journal is © The Royal Society of Chemistry 2017 |