Mechanistic insights into biomimetic carbonic anhydrase action catalyzed by doped carbon nanotubes and graphene†
Abstract
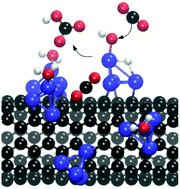
Electronic structural analyses of hydrogen terminated metal doped carbon nanotube/graphene (M-CNT/Gr, MN3-CNT/Gr, M = Ru/Rh) and ruthenium cluster decorated carbon nanotube/graphene (Ru4-CNT/Gr) were carried out for examining the biomimetic catalytic activity towards CO2 hydration reaction. The carbonic anhydrase action was followed for the reaction of CO2 with H2O resulting in a bicarbonate ion and a proton. All the catalysts were found to be active for CO2 hydration and the mechanism proved them to be biomimetic. Interconversion of CO2 to a HCO3− ion took place with five elementary steps viz. OH− formation by H2O dissociation, linear CO2 complexation, CO2 bending by nucleophilic attack of an OH− ion over CO2, HCO3− ion formation by intramolecular proton migration and HCO3− ion displacement by H2O addition. Free energy landscapes over the catalysts were developed for CO2 hydration reaction. The activation energies of H2O dissociation and CO2 bending were observed to be substantially smaller over Ru4-CNT when compared to those over the other catalysts. Ru4-CNT was found to be the best catalyst for CO2 hydration with the rate limiting step being HCO3− ion formation.


 Please wait while we load your content...
Please wait while we load your content...