Performance enhancement of high temperature SnO2-based planar perovskite solar cells: electrical characterization and understanding of the mechanism†
Abstract
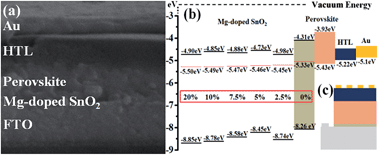
Mg doping into high temperature processed (HTP) SnO2 as an electron selective layer (ESL) significantly improves perovskite solar cell (PSC) performance including power conversion efficiency (PCE), open circuit voltage, short circuit current (JSC) and fill factor. An optimum Mg content (7.5%) affords a uniform, smooth and dense Mg-doped SnO2 film with high electron mobility and its corresponding PSC displays less hysteresis and achieves a highest steady-state PCE of 14.55%, nearly 92.8% enhancement compared to that with undoped SnO2. Electrical measurements show that suitable Mg doping dramatically reduces free electron density and substantially increases the electron mobility of pristine SnO2. The mechanism of efficiency enhancement for PSCs is proposed as follows: the low free electron density causes suppression of carrier recombination and high electron mobility facilitates fast extraction of electrons from perovskite to ESLs, contributing to an improved JSC. Impedance analysis strongly supports the proposed mechanism and reveals that the higher is the electron mobility, the higher is the electron collection efficiency, and the higher are the JSC and PCE. The HTP SnO2 with a suitable Mg content can be an excellent ESL for PSCs and might well be a suitable candidate of ESLs for CdTe, CuInGaSe and other photovoltaic devices involved in HTP treatment.

 Please wait while we load your content...
Please wait while we load your content...