Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing and reducing chirality by opposite circularly-polarized light irradiation on crystalline chiral domains consisting of nonchiral photoresponsive W-shaped liquid crystal molecules
Suk-Won
Choi
*ab and
Hideo
Takezoe
*bc
aDepartment of Advance Materials Engineering for Information and Electronics, Kyung Hee University, Yongin-shi, Gyeonggi-do 446-701, Korea. E-mail: schoi@khu.ac.kr
bDepartment of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo, 152-8552, Japan
cToyota Physical and Chemical Research Institute, 41-1 Yokomichi, Nagakute, Aichi 480-1192, Japan. E-mail: htakezoe@yf6.so-net.ne.jp
First published on 25th August 2016
Abstract
We found possible chirality enhancement and reduction in chiral domains formed by photoresponsive W-shaped molecules by irradiation with circularly polarized light (CPL). The W-shaped molecules exhibit a unique smectic phase with spontaneously segregated chiral domains, although the molecules are nonchiral. The chirality control was generated in the crystalline phase, which shows chiral segregation as in the upper smectic phase, and the result appeared to be as follows: for a certain chiral domain, right-CPL stimuli enhanced the chirality, while left-CPL stimuli reduced the chirality, and vice versa for another chiral domain. Interestingly, no domain-size change could be observed after CPL irradiation, suggesting some changes in the causes of chirality. In this way, the present system can recognize the handedness of the applied chiral stimuli. In other words, the present material can be used as a sensitive chiral-stimuli-recognizing material and should find invaluable applications, including in chiroptical switches, sensors, and memories as well as in chiral recognition.
Introduction
Since Pasteur succeeded in separating two isomers of sodium ammonium tartrate in 1848,1 chirality has been regarded as an intriguing and universal phenomenon in nature. For example, the chirality of DNA and RNA is preserved in all species.2 Furthermore, asymmetrical enantiomers can selectively recognize and interact with specific chiral molecules.3,4 Controlling chirality is also one of the hot topics in soft matter.5–7 Chiral matters exhibit unique chirality-related optical effects, such as optical rotatory dispersion and circular dichroism (CD) effects,8,9 so, are easily recognized.In the field of liquid crystal (LC) science, chirality is also one of the most active research topics.10 The most remarkable feature is the spontaneous formation of a macroscopic chiral domain (chiral segregation or reflection symmetry breaking), which was mostly observed in bent-core molecular systems.11,12 The first recognition was made in the B2 (tilted smectic) phase consisting of polar layers. The relationship between the long molecular axis, the polar direction, and the layer normal defines the chirality of each layer (layer chirality).13 The dark conglomerate (optically isotropic chiral sponge) phase14 and the B7 (polarization modulated)15 phase are other examples. Tschierske and Ungar16 reviewed reflection symmetry breaking in liquid and LCs of achiral molecules including cubic and even isotropic phases. More recently, even a new nematic phase was found to show reflection symmetry breaking, i.e., a twist-bend nematic (NTB) phase, in which bent-shaped mesogens form a heliconical structure with its pitch of a few molecular lengths.17–19 Further, a more widely studied phase from the viewpoint of chirality is the B4 (helical nanofilament: HNF) phase.20 In this phase, smectic layers form a helical structure like the twist grain boundary (TGB) phase,21 but the twist is continuous unlike TGB. The HNF has a width of about 25 nm, and a helical pitch of about 100 nm.20 The same as previous examples, the HNF phase microscopically segregates into two chiral domains with equal probabilities. Many attempts have been successfully made for controlling chirality22–26 and uniformly aligning HNFs.27,28 In these studies, chirality control and uniform alignment of HNFs are possible, if the controlling processes are conducted during the phase transition to the B4 (HNF) phase from the upper phases such as normal smectic or nematic phases. This is because the B4 phase has more or less a crystalline nature. The same as in the B4 phase, chirality control in chiral segregated states is not possible in the phases mentioned above except for the B2 phase and the SmX phase of bent-shaped twin dimers.25 In the B2 phase, polarization switching by electric-field-induced molecular rotation about its long axis but not by the rotation around the tilt cone brings about the switching of the layer chirality.29–31 In the SmX phase, similar to the B4 phase in bent-shaped twin dimers, the CD intensity was found to be changed by circularly polarized light (CPL). In this paper, we will show another example, in which chirality can be enhanced or reduced by irradiating chiral domains with CPL to chiral segregated domains.
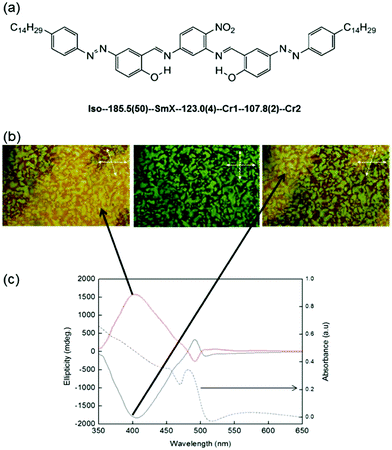
The molecule used was an achiral W-shaped molecule possessing azo-linkages at both arms. The W-shaped molecule was designed so as to have a high azobenzene content per single molecule, as well as a unique bent-core shape with a twisted molecular chirality. The molecule and the phase sequence have already been reported by Rao et al.,32 and are shown in Fig. 1(a). The SmX phase appears as a fine filament growth, and chiral domains are spontaneously formed in the SmX and the crystalline phases like in the B4 phase.32 Based on detailed experiments such as optical observations and X-ray diffraction (XRD) studies, a model structure was proposed; a concentric cylinder formed by smectic layers, on which the c-director of the W-shaped molecules form screw-like and/or polar arrangements.33 The SmX phase is distinctly different from the B4 phase, as evidenced by the texture and XRD observations. The filament sizes are quite different; this fact is based on the filament visibility under an optical microscope compared with HNFs of a nanometer scale, and different XRD diffraction widths (different coherent length) originating from the filament size difference. In the present study, we will show the enhancement and reduction of chirality that occurs depending on the handedness of the CPL used and that of the chiral domains; in a particular chiral domain, the right (left)-CPL stimuli enhanced (reduced) the chiral intensity (CD intensity), and vice versa in the opposite chiral domain. A preliminary observation was made quite a long time ago.34 The chirality enhancement and reduction were observed in a polymer film doped with W-shaped molecules. Herein, we will show that chirality control is also possible even in the crystalline phase.
Experimental
A W-shaped molecule was synthesized and supplied by Rao et al. Details of the synthesis method of the W-shaped molecule (Fig. 1(a)) used and the properties of the W-shaped molecule were described in previous papers.32,33 Sandwich cells were fabricated using fused-quartz slides without using any alignment treatments such as coating with an alignment layer or rubbing. The dimensions and thickness of the cells were approximately 1.0 × 1.0 cm2 and 2.5 μm, respectively. The W-shaped molecule was injected into the sandwich cells in the isotropic (Iso) phase. Upon cooling the cell from Iso, a smectic phase (designated as SmX) appears at 185.5 °C as an optically visible filament growth. Finally, two chiral domains were observable under decrossed polarizers in the SmX phase. Transitions to two crystalline phases (123.0 °C to Cr1 and 107.8 °C to Cr2) were observed upon further cooling. The chirality of the SmX phase was retained during the transition to the crystalline phases (Cr1 and Cr2). Irradiation was performed in Cr2 at room temperature (RT) using an Ar+ laser; the beam was first passed through a Fresnel rhomb to obtain CPL. The areas to be irradiated were selected using an aperture with a diameter of 2 mm. Irradiation was performed at the absorption band of the W-shaped molecule (488 nm). The intensity of the irradiation light was 30 mW cm−2. The two chiral domains were evaluated by means of CD spectroscopic analysis (JASCO J-720WI), while direct observations of the texture were made under a polarizing microscope (Nikon, OPTIPHOT-POL). All the evaluations were carried out at RT and atmospheric pressure.Results and discussion
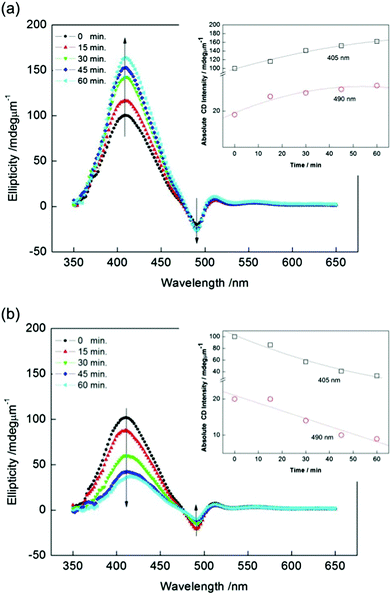
Fig. 1(b) shows polarizing optical microscope (POM) images of the domains formed at RT. As can be seen from the figure, two domains with different colors could be recognized when one of the polarizers was rotated clockwise by a few degrees. When the polarizer was rotated counterclockwise, the colors of the domains interchanged. The boundary between two chiral domains is not sharp, as previously observed in ref. 32, which is another difference from the chiral domains observed in the B4 phase. The sizes of the domains were primarily dependent on the cooling rate from the isotropic phase. Larger domains could be obtained by using a lower cooling rate.32 To obtain domains larger than a few millimeters, we performed cooling at a rate as low as 0.5 °C min−1. Fig. 1(c) shows the two CD spectra observed for the two domains. The absorption spectrum is also shown in Fig. 1(c). For each domain, two distinct CD bands of opposite signs were observed, at approximately 490 nm and 405 nm; the crossover point was approximately at 480 nm. In addition, the CD signals observed for the two domains were perfect mirror images, indicating that both domains have chiral structures with opposite senses. Thus, the different colors are due to optical rotation. We hereafter refer to the chiral domain with the positive CD intensity, observed at approximately 405 nm, as the (+) chiral domain, while the chiral domain with the negative CD intensity is referred to as the (−) chiral domain.Firstly, we show the results for the (+) chiral domain irradiated with right- and left-CPL at RT. Fig. 2(a) and (b) show the CD spectra for the (+) chiral domain after exposure for 0, 15, 30, 45 and 60 min to right- and left-CPL, respectively, at a wavelength of 488 nm at RT. The temporal variations of the absolute CD intensities (ellipticities) at 405 nm and 490 nm are also shown in the inset. As can be seen from Fig. 2(a), as the irradiation time of the right-CPL stimuli was increased, the circular anisotropy (chirality) increased markedly. In contrast, as can be seen from Fig. 2(b), as the irradiation time of the left-CPL stimuli was increased, the circular anisotropy decreased significantly. However, after irradiation for more than 60 min, the absolute CD intensity plateaued; the sign of the CD signal never changed.
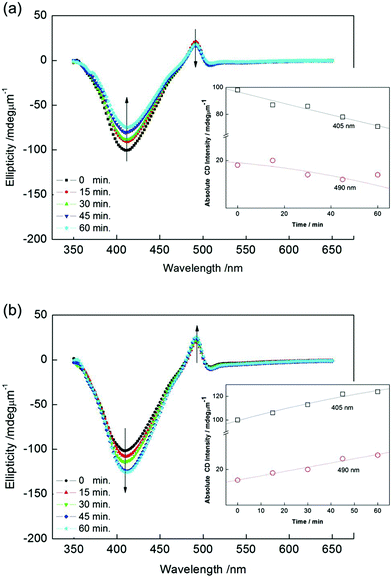
Next, we irradiated the (−) chiral domain with right- and left-CPL at RT. Fig. 3(a) and (b) show the CD spectra from the (−) chiral domain after exposure for 0, 15, 30, 45, and 60 min to right- and left-CPL, respectively. The situation is the same as in the (+) domain. The absolute CD intensity at 405 nm and 490 nm also showed a decrease and increase similar to the (+) domain (see inset). These experiments made in the (−) and (+) domains clearly indicate that the chirality is enhanced or reduced, when chiral domains are stimulated by CPL with the same and opposite handedness, respectively. In other words, chiral recognitions are possible between chiral stimuli and chiral domains formed by W-shaped molecules.
Similar phenomena were observed in polymer films containing azo linkages in the side chains,7,35 in the main chain,36 and as a dopant.34 In all these systems, the virgin samples are nonchiral (no CD activity), but chirality (CD signal) is induced, increased, decreased, and even changed its sign during prolonged right- or left-CPL stimuli. During these processes, no chiral domain nucleation was observed except for the apparent texture change suggesting molecular translation.7 In contrast, segregated chiral domains exist spontaneously, and only enhancing and reducing the chirality is possible, but conversion to the opposite chiral handedness (opposite CD signal) is not allowed in the present system. This is a distinct difference compared with the polymer case mentioned above.7,34–36 It is also important to point out that no remarkable textural changes are observed in the shape, size, and color of the chiral domains in the present system.
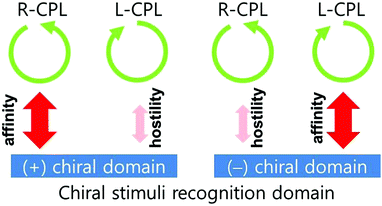
An image illustrating this phenomenon is shown in Fig. 4. Hence, the chiral domains used in this work can be regarded as sensitive chiral-stimuli-recognition materials. As shown in the figure, the right- and left-CPL stimuli affected the chiral domains differently. In the (+) chiral domain, the right (left)-CPL stimuli enhanced (reduced) the chiral intensity. In contrast, the left (right)-CPL stimuli enhanced (reduced) the chiral intensity in the opposite chiral domain.
The key question now is what is happening in the domains when the chirality is enhanced or reduced? In our previous study, an imbalance between two chiral-domain areas was successfully created using CPL irradiation during the transition to the phase with chiral segregation, resulting in almost 100% ee (enantiomeric excess). However, in the present case, the size and shape of the chiral domains do not change. Hence, the origin must be ascribed to the chiral strength change in these chiral domains. In polymeric systems, different mechanisms are proposed; (1) photo-induced mass transportation or diffusion to create chiral conformation such as a helix not only by side chains but also by main chains,7,35 and (2) exciton coupling with preferential sense in a realigned polymer backbone.34,36 The experimentally observed CD spectra were analyzed using the DeVoe polarizability model associated with the coupled oscillator method37,38 and were well simulated.34 Even for (1), exciton coupling with a helical structure must be the origin for large CD signals (a few thousand mdeg μm−1), although it is not clearly stated in these papers.7,35 In both cases, helical or some local chiral structures must be formed. For the formation process, local heating is helpful for photoisomerisation efficiency. More or less local heating occurs due to light absorption also in the present experiment. But the experiment was carried out at RT well below the Cr1–Cr2 phase transition. Therefore, the essential role is played by circularly polarized light. The two mechanisms mentioned above would apply to the present system. Actually, induced CDs are a few thousand mdeg μm−1 for (1) and a few hundred mdeg μm−1 for (2), being comparable with the present case. However, because of the different behavior between the polymer and the present cases, i.e., the absence and presence of initial chiral domains and the possible and impossible handedness change, respectively, we want to seek another mechanism.
There are two origins of chirality, i.e., layer chirality13 and molecular chirality due to twisted molecular conformation, which are actually correlated with each other.39–41 Molecular chirality gives only a negligible effect. This is clear if one considers that the melt or solution of chiral molecules exhibits a very tiny CD signal from thin samples such as a few microns. In contrast, layer chirality would exhibit a remarkable CD signal such as 1 deg μm−1 even from a few layered samples.42–44 The layer chirality is 104 times as large as the molecular chirality.43 The model structure of the SmX phase of the W-shaped molecule says, as already described in the Introduction, that smectic layers form a concentric cylinder and the W-shaped molecules are tilted from the smectic layer normal to form screw-like and/or polar arrangements.33 Hence, layer chirality does exist in the present system. According to the ESI in ref. 44, the optical rotatory power ϕ is given by many parameters such as the dielectric constant and the number of layers, the layer thickness, and the tilt angle θ. Among them, only the tilt angle and the layer thickness could be changed by CPL irradiation. Since the layer thickness change cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ is negligible, we describe ϕ as a function of θ by turning a blind eye to the dependence of light propagation with respect to the layer direction;
θ is negligible, we describe ϕ as a function of θ by turning a blind eye to the dependence of light propagation with respect to the layer direction;
ϕ ∝ sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + 9 θ + 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3θ. 3θ. | (1) |
The dependence of ϕ on θ is quite noticeable. For instance, if θ changes from 10 deg to 15 deg, ϕ increases by about 42%. This kind of tilt change may occur by CPL irradiation due to the following process. By light irradiation, photoisomerisation and relaxation between trans and cis repeatedly occur, which is promoted by local heating. Depending on right- and left-CPL, conformers with certain chirality become more and more dominant compared with the original state, where a finite amount of minor isomers exist. Since the chiral conformers and layer chirality are correlated, the tilt angle may increase when the ratio of the majority isomers against the minority ones increases. Better packing may also affect the tilt increase.
The chirality induced in polymers with side chains with azo linkages by CPL irradiation,7,35 which is known to exhibit large CD signals such as 1 deg μm−1, might be another/additional cause for the enhancement and reduction of chirality in the present case, as mentioned above. Although the phenomenon has not fully been understood yet, chiral structural formation of azo-benzene groups is suggested. To identify the real cause, microscopic structural analysis methods such as freeze-fractured transmission electron microscopy and atomic force microscopy are necessary.
Conclusions
Enhancement and reduction of chiral strength (CD) were observed without changing the domain texture using CPL irradiation on chiral domains in the crystalline phase formed by nonchiral and photoactive W-shaped molecules. A possible explanation was given for this phenomenon. This observation suggests the possibility that the chiral domains formed by the W-shaped molecules can recognize the chirality of the applied stimuli. Instead of CPL irradiation, other chiral stimuli including other chiral molecules may be tried for detecting selective chiral interactions, leading to sensing devices for chiral compounds. Such sensitive chiral-stimuli-recognizing materials should find wide use in a range of applications, including in chiroptical switches, sensors, and memories, as well as in chiral recognition.Acknowledgements
The authors wish to thank Prof. N. V. S. Rao, and Prof. M. K. Paul from Assam University, India, for supplying valuable W-shaped molecules. This work was supported by the Basic Science Research Program (NRF-2013R1A1A2061996) through the National Research Foundation of Korea (NRF).Notes and references
- L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed.
- S. F. Mason, Nature, 1984, 311, 19 CrossRef CAS PubMed.
- J. E. Hein and D. G. Blackmond, Acc. Chem. Res., 2012, 45, 2045 CrossRef CAS PubMed.
- D. F. Williams, Biomaterials, 2008, 29, 2941 CrossRef CAS PubMed.
- M. Avalos, R. Babiano, P. Cintas, J. L. Jimenez, J. C. Palacios and L. D. Barron, Chem. Rev., 1998, 98, 2391 CrossRef CAS PubMed.
- A. Ohira, K. Okoshi, M. Fujiki, M. Kunitake, M. Naito and T. Hagihara, Adv. Mater., 2004, 16, 1645 CrossRef CAS; K. Kim, S.-T. Hur, S. Kim, S.-Y. Jo, B. Lee and M. H. Song, J. Mater. Chem. C, 2015, 3, 5383 RSC.
- G. Iftime, F. L. Labarthet, A. Natansohn and P. Rochon, J. Am. Chem. Soc., 2000, 122, 12646 CrossRef CAS; K. Kim, S. Kim, S.-Y. Jo and S.-W. Choi, J. Inf. Disp., 2015, 16, 155 CrossRef.
- S. Superchi, E. Giorgio and C. Rosini, Chirality, 2004, 16, 422 CrossRef CAS PubMed.
- A. Rodger and B. Norden, Circular Dichroism and Linear Dichroism, Oxford Univ. Press, Oxford, 1997 Search PubMed.
- H. Takezoe, Top. Curr. Chem., 2012, 318, 303 CrossRef CAS PubMed; K. Kim, H. Kim, S.-Y. Jo, F. Araoka, D. K. Yoon and S.-W. Choi, ACS Appl. Mater. Interfaces, 2015, 7, 22686 Search PubMed.
- H. Takezoe and Y. Takanishi, Jpn. J. Appl. Phys., 2006, 45, 597 CrossRef CAS.
- R. A. Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907 RSC.
- D. R. Link, G. Natale, R. Shao, J. E. Maclennan, N. A. Clark, K. Eva and D. M. Walba, Science, 1997, 278, 1924 CrossRef CAS PubMed.
- L. E. Hough, M. Spannuth, M. Nakata, D. A. Coleman, C. D. Jones, G. Dantlgraber, C. Tschierscke, J. Watanabe, E. Korblova, D. M. Walba, J. E. Maclennan, M. A. Glaser and N. A. Clark, Science, 2009, 325, 452 CrossRef CAS PubMed.
- D. A. Coleman, J. Fernsler, N. Chattham, M. Nakata, Y. Takanishi, E. Korblova, D. R. Link, R.-F. Shao, W. G. Jang, J. E. Maclennan, O. Mondainn-Monval, C. Boyer, W. Weissflog, G. Pelzl, L.-C. Chien, J. Zasadzinski, J. Watanabe, D. M. Walba, H. Takezoe and N. A. Clark, Science, 2003, 301, 1204 CrossRef CAS PubMed.
- C. Tschierske and G. Ungar, ChemPhysChem, 2016, 17, 9 CrossRef CAS PubMed.
- V. Borshch, Y.-K. Kim, J. Xiang, M. Gao, A. Jaki, V. P. Panov, J. K. Vij, C. T. Imrie, M. G. Tamba, G. H. Mehl and O. D. Lavrentovich, Nat. Commun., 2013, 4, 2635 CAS.
- D. Chen, J. H. Porada, J. B. Hooper, A. Klittnick, Y. Shen, M. R. Tuchband, E. Korblova, D. Bedrov, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Proc. Natl. Acad. Sci. U. S. A., 2013, 1101, 15931 CrossRef PubMed.
- C. Meyer, G. R. Luckhurst and I. Dozov, Phys. Rev. Lett., 2013, 111, 067801 CrossRef CAS PubMed.
- L. E. Hough, H. T. Jung, D. Kruerke, M. S. Heberling, M. Nakata, C. D. Jones, D. Chen, D. R. Link, J. Zasadzinski, G. Heppke, J. P. Rabe, W. Stocker, E. Korblova, D. M. Walba, M. A. Glaser and N. A. Clark, Science, 2009, 325, 456 CrossRef CAS PubMed.
- J. W. Goodby, M. A. Waugh, S. M. Stein, E. Chin, R. Pindak and J. S. Patel, Nature, 1989, 337, 449 CrossRef CAS.
- S.-W. Choi, T. Izumi, Y. Hoshino, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Angew. Chem., Int. Ed., 2006, 45, 1382 CrossRef CAS PubMed.
- S.-W. Choi, S. Kang, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Angew. Chem., Int. Ed., 2006, 45, 6503 CrossRef CAS PubMed.
- G. Lee, R. J. Carlton, F. Araoka, N. L. Abbott and H. Takezoe, Adv. Mater., 2013, 25, 245 CrossRef CAS PubMed.
- T. Ueda. S. Masuko, F. Araoka, K. Ishikawa and H. Takezoe, Angew. Chem., Int. Ed., 2013, 52, 6863 CrossRef PubMed.
- Y. Hoshino, S.-W. Choi, T. Izumi, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Mol. Cryst. Liq. Cryst., 2007, 465, 153 CrossRef CAS.
- D. K. Yoon, Y. Yi, Y. Shen, E. D. Korblova, D. M. Walba, I. I. Samalyukh and N. A. Clark, Adv. Mater., 2011, 23, 1962 CrossRef CAS PubMed.
- F. Araoka, G. Sugiyama, K. Ishikawa and H. Takezoe, Adv. Funct. Mater., 2013, 23, 2701 CrossRef CAS.
- M. W. Schroder, S. Diele, G. Pelzl and W. Weissflog, ChemPhysChem, 2004, 5, 99 CrossRef PubMed.
- W. Weissflog, U. Dunemann, M. W. Schroder, S. Diele, G. Pelzl, H. Kresse and S. Grande, J. Mater. Chem., 2005, 15, 939 RSC.
- M. Nakata, R.-F. Shao, J. E. Maclennan, W. Weissflog and N. A. Clark, Phys. Rev. Lett., 2006, 96, 067802 CrossRef CAS PubMed.
- N. V. S. Rao, M. K. Paul, I. Miyake, Y. Takanishi, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2003, 13, 2880 RSC.
- I. Miyake, Y. Takanishi, N. V. S. Rao, M. K. Paul, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2005, 15, 4688 RSC.
- S.-W. Choi, N. Y. Ha, K. Shiromo, N. V. S. Rao, M. K. Paul, T. Toyooka, S. Nishimura, J. W. Wu, B. Park, Y. Takanishi, K. Ishikawa and H. Takezoe, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 021702 CrossRef PubMed.
- Y. Wu, A. Natansohn and P. Rochon, Macromolecules, 2004, 37, 6801 CrossRef CAS.
- S.-W. Choi, T. Fukuda, Y. Takanishi, K. Ishikawa and H. Takezoe, Jpn. J. Appl. Phys., 2006, 45, 447 CrossRef CAS.
- H. DeVoe, J. Phys. Chem., 1971, 75, 1509 CrossRef CAS PubMed.
- S. Superchi, E. Giorgio and C. Rocini, Chirality, 2004, 16, 422 CrossRef CAS PubMed.
- S.-W. Choi, S. Kawauchi, N. Y. Ha and H. Takezoe, Phys. Chem. Chem. Phys., 2007, 9, 3671 RSC.
- H. Niwano, M. Nakata, J. Thisayukta, D. R. Link, H. Takezoe and J. Watanabe, J. Phys. Chem. B, 2004, 108, 14889 CrossRef CAS.
- M. Zennyoji, Y. Takanishi, K. Ishikawa, J. Thisayukta, J. Watanabe and H. Takezoe, Mol. Cryst. Liq. Cryst., 2001, 366, 693 CrossRef CAS.
- J. Ortega, C. L. Folcia, J. Etxebarria, N. Gimeno and M. B. Ros, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 011707 CrossRef CAS PubMed.
- L. E. Hough and N. A. Clark, Phys. Rev. Lett., 2005, 95, 107802 CrossRef CAS PubMed.
- J. Matraszek, N. Topnani, N. Vaupotic, H. Takezoe, J. Mieczkowski, D. Pociecha and E. Gorecka, Angew. Chem., Int. Ed., 2016, 55, 3468 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |