Chemical modification of starch and its application as an adsorbent material
Abstract
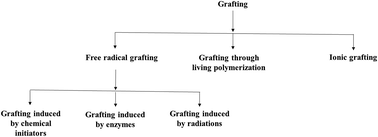
Starch is a biopolymer of plant origin which is cheap abundant and has many applications in food and non-food industries. However, in the native form, its applications are limited due to shortcomings, such as loss of viscosity and thickening power upon cooking and storage, retrogradation characteristics and absence of certain groups responsible for a particular function, etc. So, in order to reduce its limitations and improve its applications, modification of starch is necessary. It can be modified by several ways like chemical modification, physical modification and genetic modification but the most important one is the chemical modification. In this review, we selected the published data related to the chemical modification like grafting, cross-linking, esterification, etherification and dual modification of starch and application of modified starch for the adsorption of organic dyes and heavy metals from water.

 Please wait while we load your content...
Please wait while we load your content...