Synthesis of 2,4,5-trisubstituted imidazoles, quinoxalines and 1,5-benzodiazepines over an eco-friendly and highly efficient ZrO2–Al2O3 catalyst†
N. Thimmarajuab and
S. Z. Mohamed Shamshuddin*ab
aChemistry Research Laboratory, HMS Institute of Technology, Tumkur-572104, Karnataka, India. E-mail: mohamed.shamshuddin@gmail.com; Tel: +91-9844718742
bResearch and Development Center, Bharathiar University, Coimbatore, India
First published on 16th June 2016
Abstract
ZrO2–Al2O3 containing 80 mol% of ZrO2 was prepared by solution combustion, impregnation and precipitation methods. All the prepared catalytic materials were characterized by PXRD, NH3-TPD, N2 adsorption isotherms, FTIR, TEM, SEM, EDAX and ICP-OES techniques. These catalytic materials were used in a rapid, simple, versatile and efficient synthesis of 2,4,5-trisubstituted imidazoles under solvent-free conditions at moderate temperature in shorter reaction time (20 min). This is achieved by three-component cyclo-condensation of benzil, aldehydes and ammonium acetate in excellent yields. The derivatives of quinoxalines and 1,5-benzodiazepines were synthesized effectively by cyclo-condensation of 1,2-diamine with α-diketones and carbonyl compound with O-phenylenediamines respectively over ZrO2–Al2O3 at moderate temperature (80 °C) for the reaction time of 20–40 min in presence of ethanol as a solvent. The present method is experimentally simple, non-toxic and involves inexpensive reagents, clean reaction pathways and an eco-friendly catalyst. The catalytic materials used can be easily separated from the reaction mixture and reused for several reaction cycles without much loss of catalytic activity.
1. Introduction
Metal oxide and mixed metal oxide based solid acid catalysts are most important and widely employed in the largest family of heterogeneous catalysts because they utilize both acid–base and redox properties.1–4 Among various metal oxides, ZrO2 and Al2O3 have been widely used in both acid and base catalyzed reactions in the field of heterogeneous catalysis as catalysts as well as catalytic supports.5–7 The Al2O3–ZrO2 based catalysts were found to exhibit better catalytic properties than either Al2O3 or ZrO2 and are attracting considerable interest because of their potential use as catalysts.8–10 Therefore, ZrO2–Al2O3 has been used in many catalytic processes involving liquid, vapour and gas phase reactions.Multi-component reactions (MCRs) are powerful and outstanding tool in generating products in medicinal chemistry and modern organic synthesis because MCRs are one-pot process is wherein three or more components are assemble and produce product with high selectivity and high atom efficiency.11,12 MCRs have great contribution and considerable attention since they are performed without the need to isolate any intermediate and save both raw materials and energy and also reduce time.13,14 MCRs find great interest in the synthesis of important and complex organic molecules from simple and readily available starting materials in a single synthetic operation.12,15
N-Heterocyclic compounds represent one of the most valuable building blocks for biologically active molecules, drugs and functional materials.16 Naturally occurring substituted imidazoles as well as synthetic derivatives are most important class of heterocyclic compounds and play an important role in chemical and biological system.17,18 The imidazole and its derivatives are used as antibacterial agent, fungicides and herbicides, antitumor and anti-inflammatory agents, plant growth regulators and therapeutic agents.19–24 Various substituted imidazoles act as inhibitor of B-Raf kinase, p38 MAP kinase and glucagon receptors.25–27 They are also useful building blocks for the synthesis of other classes of compounds. Therefore, the organic synthesis of these imidazole derivatives has a significant impact on synthetic organic and medicinal chemistry.
A number of methods have been developed for the synthesis of 2,4,5-trisubstistuted imidazoles. The best reported route for the synthesis of 2,4,5-trisubstistuted imidazoles is three components cyclo-condensation of benzil, aldehyde and ammonium acetate in the presence of an acid catalyst. There are several methods available to synthesize imidazole derivatives over microwaves, L-proline, acetic acid, ionic liquids, tetrabutylammonium bromide, molecular iodine, montmorillonite K 10, zeolite, nano sulfated zirconia, heteropolyacids, CAN (ceric ammonium nitrate), silica gel/NaHSO4, K5CoW12O40–3H2O, FeCl3·6H2O, silica sulphuric acid, NiCl2·6H2O/Al2O3, ZrCl4, etc.28–34
Quinoxaline derivatives are an important class of benzo heterocyclic compounds, since they have a wide area of biological activities such as anti-HIV, anti-viral, anti-diabetic, anti-fungal, anti-bacterial, anti-cancer, anti-depression, etc.35,36 Numerous methods are available for the synthesis of quinoxaline derivatives which involve cyclo-condensation of 1,2-diamine with α-diketones using MnO2, POCl3, zeolites, iodine, CAN, etc.37,38
Benzodiazepine and its derivatives are another interesting class of heterocyclic compounds, as they are used as drugs, anti-anxiety, anti-convulsant, sedative, analgesic, anti-depressive, hypnotic agents, etc.39,40 The common procedure for the synthesis of 1,5-benzodiazepines is the condensation between carbonyl compound and O-phenylenediamines. Few methods have been reported with reagents such as BF3·OEt2, InBr3, polyphosphoric acid–SiO2, sulfated zirconia, Yb(OTf)3, Sc(OTf)3, molecular iodine, acetic acid, ionic liquids, etc.41,42
Some of these synthetic methods suffer from one or more serious drawbacks such as harsh reaction conditions, moisture-sensitive metallic reagents, laborious and complex work-up and purification, poor yields, significant amount of waste materials, prolonged time periods, high temperatures, occurring side reactions and use of hazardous and often expensive acid catalysts. Moreover, the synthesis of these heterocyclic compounds has been usually carried out in presence of polar solvents like N,N-dimethylformamide, acetic acid and dimethylsulfoxide leading to complex isolation and recovery procedure.
In the present work, an attempt has been made for the synthesis of 2,4,5-trisubstituted imidazoles, quinoxalines and 1,5-benzodiazepines at moderate temperature in a shorter reaction time over ZrO2–Al2O3 as a simple, low cost, eco-friendly, reusable and highly efficient catalyst.
2. Experimental
2.1. Chemicals
Zirconyl nitrate hydrate, aluminium nitrate nonahydrate and urea were supplied by M/S LOBA Chemie, India. Substituted benzil, aromatic aldehydes, ketones, α-diketones and O-phenylenediamines were supplied by Sigma Aldrich or Alfa-Aesar and the solvents were supplied by ChemLabs, India.2.2. Preparation of ZrO2–Al2O3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous ammonia solution was added drop wise with constant stirring until precipitation is complete. Thus obtained precipitate was filtered and dried in hot air oven at 120 °C for 12 h.
1 aqueous ammonia solution was added drop wise with constant stirring until precipitation is complete. Thus obtained precipitate was filtered and dried in hot air oven at 120 °C for 12 h.These prepared samples were finely powdered and calcined in a muffle furnace at 550 °C for 5 h before their use as a catalytic material. ZrO2–Al2O3 prepared by solution combustion method was abbreviated as ZA-SCM, impregnation method as ZA-IMG and precipitation as ZA-PPT.
2.3. Characterization of catalytic materials
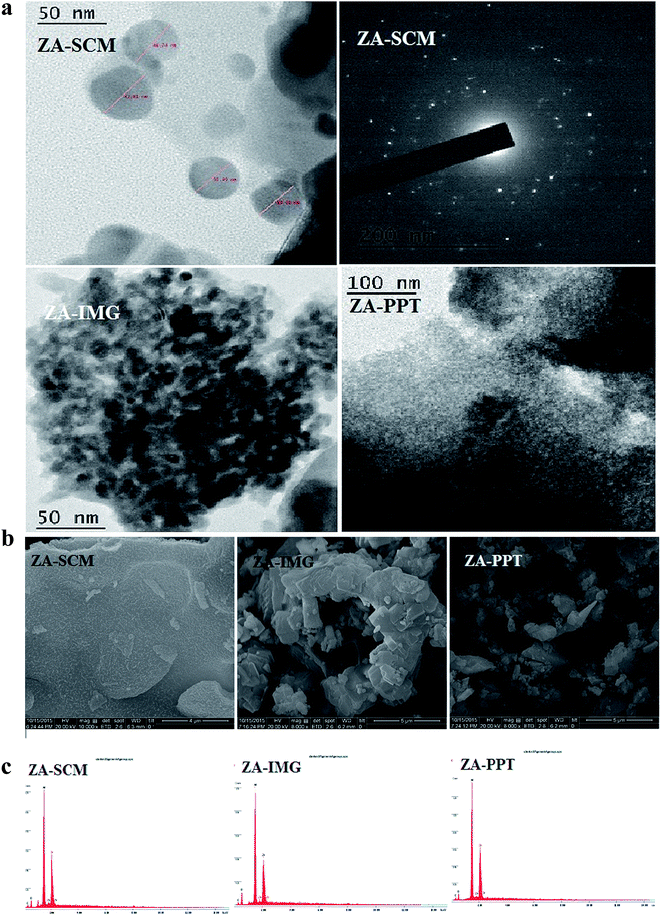
PXRD patterns of these solid acids were obtained from X'pert Pro Philips diffractometer equipped in a Ni filtered Cu-Kα radiation with λ = 1.5418 Å using a graphite crystal monochromator with a scanning range 20–70°. NOVA 1000 Quanta chrome high-speed gas sorption analyzer instrument was used to measure the specific surface area of all the prepared catalytic materials. The values of total surface acidity (TSA) of the catalytic materials were obtained from NH3-TPD method using Mayura TPD unit. The FT-IR spectrums were recorded with 4 cm−1 resolution using a Nicolet IR200 FT-IR spectrophotometer in the range 400–4000 cm−1 using KBr as a standard reference. TEM images of all the prepared catalytic materials were obtained from PHILIPS CM200 electron microscope at an acceleration voltage of 20–200 kV. SEM images and EDAX of all the prepared catalytic materials were obtained from JEOL JXA-8530F microscope. The amount of zirconia present in all the solid acid catalysts was estimated by Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) analysis technique using Thermo-iCAP 6000 Series instrument.2.4. Catalytic activity studies of ZrO2–Al2O3
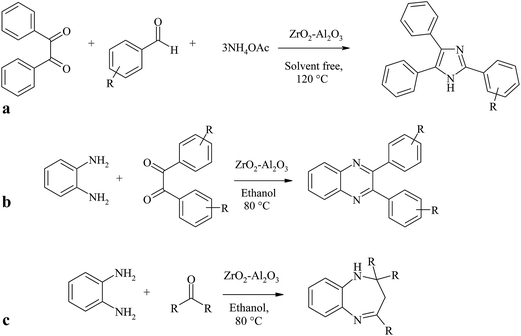
To the stirred suspension of benzil (1.43 mmol), aldehydes (1.43 mmol), ammonium acetate (4.29 mmol) in a 5 mL round bottomed flask fitted with water cooled condenser, catalytic material (0.03 g of ZrO2–Al2O3) was added and the resulting mixture was heated at 120 °C. The progress of the reaction was monitored by thin layer chromatography (TLC). After the completion of the reaction, the reaction mixture was cooled, the residue was dissolved in acetone and the resulting solution was filtered, residue was washed with acetone to recover the catalyst. Filtrate was poured to 100 mL of ice cold water, obtained precipitate was filtered, washed with hexane and dried to get the desired product. Thus obtained product was then characterized by melting point (melting points were measured on a Büchi B-540 capillary melting point apparatus and are uncorrected), 1H NMR spectroscopy (Bruker, 400 MHz) and LC-MS (Varian) techniques.Quinoxalines and 1,5-benzodiazepines were synthesized by stirring the mixture of 1,2-dicarbonyl (1.43 mmol), O-phenylenediamines (1.43 mmol), 0.03 g of ZA-IMG and ethanol (10 mL) at 80 °C and the mixture of O-phenylenediamines (1.43 mmol), ketones (2.86 mmol), 0.03 g of ZA-IMG and ethanol (10 mL) at 80 °C respectively and the progress of the reactions were monitored by TLC.
2.5. Reusability study of ZrO2–Al2O3 catalytic materials
After each reaction cycle of cyclo-condensation reaction, ZrO2–Al2O3 catalytic material was filtered from the reaction mixture, washed with acetone, dried at 120 °C for 1 h and calcined at 550 °C for 0.5 h. Thus reactivated ZrO2–Al2O3 catalytic material was used in the next cycle of the synthesis of imidazole under similar reaction conditions.The reactivation and reusability of the used ZrO2–Al2O3 catalytic material was repeated for 6 reaction cycles by following the procedure as described above (Section 2.4).
3. Results and discussion
3.1. Characterization of catalytic materials
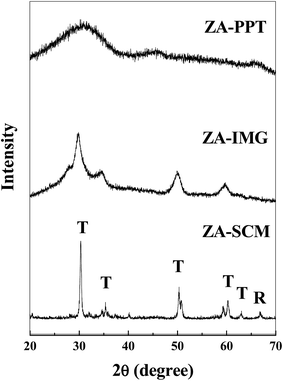
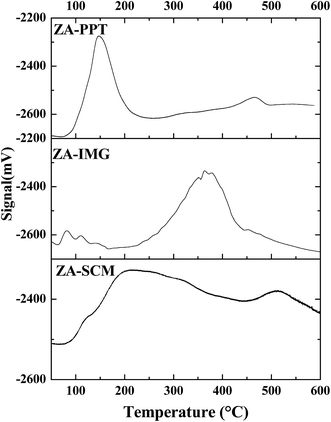
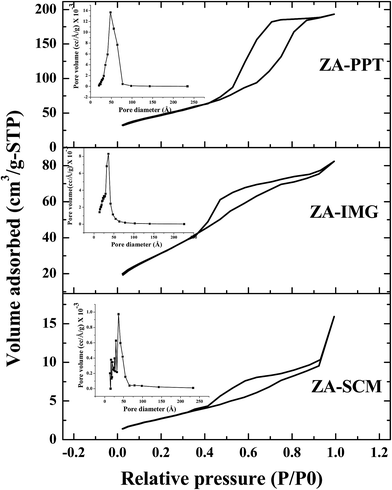
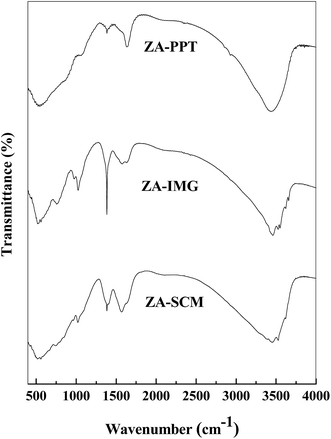
All the prepared ZrO2–Al2O3 catalytic materials were characterized for their physico-chemical properties such as PXRD, NH3-TPD, N2 adsorption isotherms, FTIR, TEM, SEM, EDAX and ICP-OES techniques.| Sl. No. | Catalytic materials | Surface area (m2 g−1) | TSA (mmol g−1) | Pore diameter (Å) | Pore volume (cm3 g−1) |
|---|---|---|---|---|---|
| 1 | ZA-SCM | 10.607 | 1.33 | 57.84 | 0.016 |
| 2 | ZA-IMG | 119.68 | 1.52 | 39.95 | 0.119 |
| 3 | ZA-PPT | 168.98 | 1.27 | 68.97 | 0.291 |
The ZA-IMG catalytic material was comparatively found to possess higher total surface acidity than ZA-SCM or ZA-PPT. This may be due to tetragonal crystalinity. It is very interesting to observe that the total surface acidity is not dependent on the surface area. Desorption peaks of weak, medium, strong and very strong acid sites of ZrO2–Al2O3 catalytic materials are observed in Fig. 2.
3.2. Catalytic activity studies
As a test reaction, the three-component cyclo-condensation of benzil, aldehydes and ammonium acetate with ZrO2–Al2O3 catalytic materials under solvent-free conditions was carried out at optimized reaction conditions at 120 °C (Scheme 1).51 All the three ZrO2–Al2O3 catalytic materials used in the present work were active in this reaction.When the reaction was carried out in the absence of ZrO2–Al2O3 catalytic material, there was a negligible amount of formation of the reaction product. This indicates that the cyclo-condensation is a catalyzed reaction.
3.3. Effect of nature of ZrO2, Al2O3 and ZrO2–Al2O3 on the yield 2,4,5 triphenyl-1H-imidazole
In an initial study, the reactions were carried out with 0.03 g of ZA-SCM, ZA-IMG and ZA-PPT in cyclo-condensation reaction of benzil (1.43 mmol), benzaldehyde (1.43 mmol) and ammonium acetate (4.29 mmol). It is interesting to note that a maximum yield (99.2%) of 2,4,5 triphenyl-1H-imidazole (Table 2, entry 1) was observed for ZA-IMG under solvent free condition at 120 °C in 20 min. 95.1% and 89.9% of 2,4,5 triphenyl-1H-imidazole was obtained with ZA-SCM and ZA-PPT catalytic materials respectively. There is no particular correlation observed between the catalytic activity and the surface area. This indicates that the distribution of catalytic active species on the surface plays significant role than the surface area of the catalysts.| Sl. No. | Catalytic materials | TSA (mmol g−1) | Yield of 2,4,5-triphenyl-1H-imidazole (%) |
|---|---|---|---|
| 1 | Al2O3 | 0.49 | 60.0 |
| 2 | ZrO2 | 0.43 | 53.6 |
| 3 | 10ZA | 0.65 | 65.2 |
| 4 | 20ZA | 0.76 | 73.5 |
| 5 | 40ZA | 1.05 | 80.7 |
| 6 | ZA-SCM (80ZA) | 1.33 | 95.1 |
| 7 | ZA-IMG | 1.52 | 99.2 |
| 8 | ZA-PPT | 1.27 | 89.9 |
In order to compare the catalytic activity of ZA-SCM, ZA-IMG and ZA-PPT with similar type of catalytic materials in cyclo-condensation reaction of benzil (1.43 mmol), benzaldehyde (1.43 mmol) and ammonium acetate (4.29 mmol), the reactions were carried out in presence of 0.03 g of Al2O3, ZrO2, 10 mol% ZrO2–Al2O3 (10 ZA), 20 mol% ZrO2–Al2O3 (20 ZA), 40 mol% ZrO2–Al2O3 (40 ZA) and 80 mol% ZrO2–Al2O3 (80 ZA) or ZA-SCM prepared by SCM using urea as a fuel and the results are shown in Table 2. When the catalytic activity of these catalytic materials was compared ZA-IMG showed better yield of 2,4,5 triphenyl-1H-imidazole derivatives than other catalytic materials used for the present study. The better catalytic activity of ZA-IMG can be due to its higher surface acidity when compared to other similar metal oxides.52
Therefore, ZA-IMG catalyst was chosen for further studies for the synthesis of different 2,4,5-tri substituted imidazoles.
3.4. Synthesis of different 2,4,5 tri substituted imidazoles over ZA-IMG
The cyclo-condensation reaction of benzil (1.43 mmol) with various aldehydes (1.43 mmol) bearing electron-donating groups (methyl or diethyl amine) or electron-withdrawing groups (halides) at para or meta position and ammonium acetate (4.29 mmol) was carried out in presence of 0.03 g of ZA-IMG catalytic material at 120 °C and the progress of the reactions were monitored by TLC. Workup procedure for the reaction and characterization of reaction products has been discussed in Section 2.4. The reaction time and isolated yield (%) of desired reaction products are listed in Table 3 and the spectral data of isolated products are presented in Appendix-1.| Entry | Aldehyde | Reaction product | Reaction time (min) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.03 g of ZA-IMG at 120 °C, benzil (1.43 mmol), aldehyde (1.43 mmol) and ammonium acetate (4.29). | ||||
| 1 | 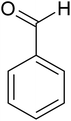 |
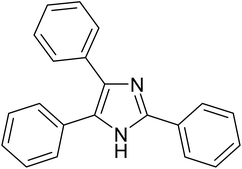 |
20 | 99.2 |
| 2 | 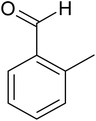 |
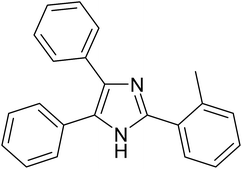 |
20 | 97.6 |
| 3 | 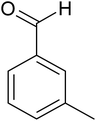 |
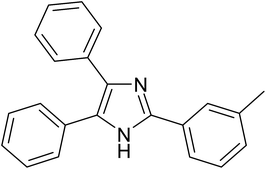 |
20 | 98.0 |
| 4 | 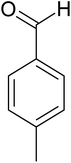 |
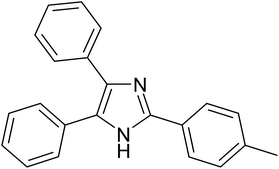 |
20 | 97.3 |
| 5 | 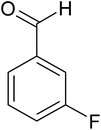 |
 |
20 | 96.5 |
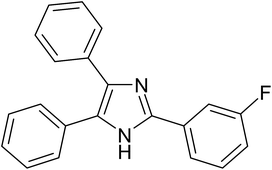
| 6 | 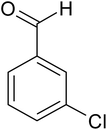 |
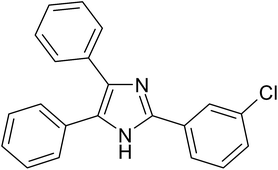 |
20 | 96.2 |
| 7 | 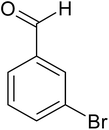 |
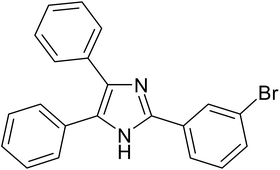 |
20 | 95.9 |
| 8 | 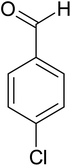 |
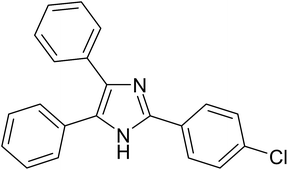 |
20 | 97.5 |
| 9 | 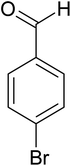 |
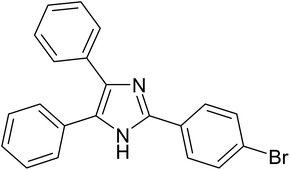 |
20 | 97.7 |
| 10 |  |
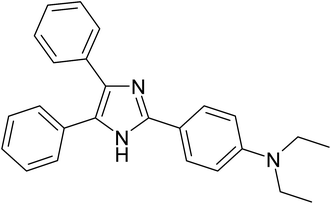 |
20 | 97.1 |
3.5. Synthesis of different quinoxalines over ZA-IMG
A mixture of 1,2-dicarbonyl (1.43 mmol), O-phenylenediamine (1.43 mmol), 0.03 g of ZA-IMG and ethanol (10 mL) was stirred at 80 °C and the progress of the reactions were monitored by TLC.37c After completion of the reaction, the reaction mixture was filtered to recover the catalytic material, filtrate was evaporated in vacuum to afford crude, which was purified by silica gel column chromatography using suitable solvent (ethyl acetate in hexane) to obtained desired product of quinoxaline. The reaction time and isolated yield (%) of desired reaction products are listed in Table 4 and the spectral data of isolated products are presented in Appendix-1.| Entry | α-Diketone | Reaction product | Reaction time (min) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.03 g of ZA-IMG at 80 °C, α-diketone (1.43 mmol), O-phenylenediamine (1.43 mmol) and ethanol (10.0 mL). | ||||
| 1 |  |
 |
20 | 90.3 |
| 2 |  |
 |
20 | 91.6 |
| 3 |  |
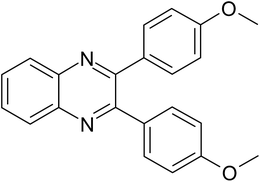 |
20 | 91.0 |
3.6. Synthesis of different 1,5-benzodiazepines over ZA-IMG
A mixture of O-phenylenediamine (1.43 mmol), ketone (2.86 mmol), 0.03 g of ZA-IMG and ethanol (10 mL) was stirred at 80 °C and the progress of the reactions were monitored by TLC.41c After completion of reaction, the reaction mixture was filtered to recover the catalytic material, filtrate was evaporated in vacuum to afford crude, which was purified by silica gel column chromatography using suitable solvent (ethyl acetate in hexane) to afford desired product (1,5-benzodiazepine). The reaction time and isolated yield (%) of desired reaction products are listed in Table 5 and the spectral data of isolated products are presented in Appendix-1.| Entry | Ketone | Reaction product | Reaction time (min) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.03 g of ZA-IMG at 80 °C, O-phenylenediamine (1.43 mmol), ketone (2.86 mmol) and ethanol (10.0 mL). | ||||
| 1 | 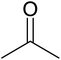 |
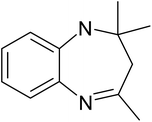 |
30 | 95.7 |
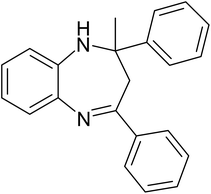
| 2 | 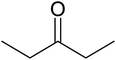 |
 |
30 | 96.1 |
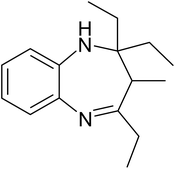
| 3 |  |
 |
40 | 92.3 |
Appendix-1
Spectral data of synthesized esters (refer to Tables 3–5)
Effect of re-usability of ZA-IMG catalytic materials on the yield of 2,4,5-triphenyl-1H-imidazole
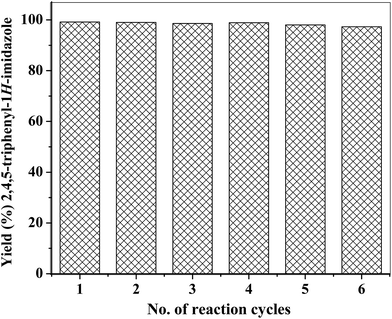
In order to study the reusability of ZA-IMG catalyst, the reaction was carried out between benzil, benzaldehyde and ammonium acetate in presence of 0.03 g of ZA-IMG at 120 °C for 20 min. The catalyst (ZA-IMG) was recovered after the each cycle of the reaction, followed by washing with acetone, drying in an oven at 120 °C for 1 h. Thus thermally regenerated ZA-IMG was re-used in the next reaction cycle. Such studies were carried out for 6 consecutive reaction cycles and the results are presented in Fig. 6. It can be seen from the figure that, not much change in yield of 2,4,5-triphenyl-1H-imidazole could be observed. Hence, ZA-IMG catalyst could be reused at least for six or more reaction cycles without much loss of its catalytic activity. PXRD and FTIR patterns of the ZA-IMG obtained after 6th reaction cycle are showed in Fig. S1 and S2† respectively and not much change in the total surface acidity (1.52 mmol g−1) of the ZA-IMG after 6 reaction cycles was observed.4. Conclusion
In conclusion, the authors of this article have prepared an efficient and green ZrO2–Al2O3 catalytic material by following different methods. ZrO2–Al2O3 prepared by impregnation method possesses comparatively high surface acidity than ZrO2–Al2O3 prepared by either solution combustion or precipitation methods. ZrO2–Al2O3 prepared by impregnation method could effectively catalyze the cyclo-condensation of benzil, different aldehydes with ammonium acetate, carbonyl compound with O-phenylenediamines and 1,2-diamine with α-diketones with excellent yield of desired products such as 2,4,5-trisubstituted imidazoles, quinoxalines and 1,5-benzodiazepines respectively at moderate temperature in shorter reaction time under solvent free conditions. ZrO2–Al2O3 catalytic materials could be recovered and reused for at least six reaction cycles without much loss of its activity.Acknowledgements
The authors thank the VGST, GoK (GRD-375/2014-15) for the part financial support. Authors also thank the authorities of IISc, Bangalore for SEM, TEM, 1H NMR, LCMS and ICP-OES analysis. The authors are thankful to the department of chemistry, St. Joseph's college, Bangalore for PXRD and FT-IR analysis.References
- T. M. Miller and V. H. Grassian, J. Am. Chem. Soc., 1995, 117(44), 10969 CrossRef CAS.
- (a) N. Pernicone, F. Lazzerin, G. Liberti and G. Lanzavecchia, J. Catal., 1969, 14(4), 391 CrossRef CAS; (b) N. Thimmaraju, S. Z. M. Shamshuddin, S. R. Pratap and Venkatesh, J. Mol. Catal. A: Chem., 2014, 391, 55 CrossRef CAS.
- (a) A. V. Biradar, S. B. Umbarkar and M. K. Dongare, Appl. Catal., A, 2005, 285, 190 CrossRef CAS; (b) A. P. Amrute, A. Bordoloi, N. Lucas, K. Palraj and S. B. Halligudi, Catal. Lett., 2008, 126, 286 CrossRef CAS.
- (a) E. L. S. Ngee, Y. Gao, X. Chen, T. M. Lee, Z. Hu, D. Zhao and N. Yan, Ind. Eng. Chem. Res., 2014, 53, 14225 CrossRef CAS; (b) N. Thimmaraju, S. Z. M. Shamshuddin, S. R. Pratap and K. Raja, RSC Adv., 2015, 5, 99517 RSC.
- L. E. Davies, N. A. Bonini, S. Locatelli and E. E. Gonzo, Lat. Am. Appl. Res., 2005, 35, 23 CAS.
- B. M. Reddy, K. V. R. Chary, B. Rama Rao, V. S. Subrahmanyam and C. S. Sunandanat, Polyhedron, 1986, 5(112), 191 CrossRef CAS.
- H. Pines and W. O. Haag, J. Am. Chem. Soc., 1960, 82, 2471 CrossRef CAS.
- (a) Y. X. Hao, J. S. Li, X. J. Yang, X. Wang and L. D. Lu, J. Mater. Sci. Eng. A, 2004, 367, 243 CrossRef; (b) G. V. Sagar, P. V. R. Rao, C. S. Srikanth and K. V. R. Chary, J. Phys. Chem. B, 2006, 110, 13881 CrossRef CAS PubMed.
- J. D'Souza and N. Nagaraju, Indian J. Chem. Technol., 2004, 11, 401 Search PubMed.
- L. Har, J. Am. Ceram. Soc., 1990, 6, 329 Search PubMed.
- (a) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (b) C. C. A Cariou, G. J. Clarkson and M. Shipman, J. Org. Chem., 2008, 73, 9762 CrossRef PubMed.
- S. Samai, G. H. Nandi, P. Singh and M. S. Singh, Tetrahedron, 2009, 65, 10155 CrossRef CAS.
- R. R. Nagawade and D. B. Shinde, Acta Chim. Slov., 2007, 54, 642 CAS.
- K. Sivakumar, A. Kathirvel and A. Lalitha, Tetrahedron Lett., 2010, 51, 3018 CrossRef CAS.
- (a) L. Weber, Drug Discovery Today, 2002, 7, 143 CrossRef CAS PubMed; (b) S. H. Reza, R. Mohammed and A. Kobra, Chin. J. Chem., 2011, 29, 1635 CrossRef.
- (a) X. Gao, X. Chen, J. Zhang, W. Guo, F. Jin and N. Yan, ACS Sustainable Chem. Eng., 2016 DOI:10.1021/acssuschemeng.6b00767; (b) X. Chen, S. L. Chew, F. M. Kerton and N. Yan, Green Chem., 2014, 16, 2204 RSC.
- T. N. Doman, S. L. McGovern, B. J. Witherbee, T. P. Kasten, R. Kurumbail, W. C. Stallings, D. T. Connolly and B. K. Shoichet, J. Med. Chem., 2002, 47, 2213 CrossRef.
- J. G. Lambardino and E. H. Wiseman, J. Med. Chem., 1979, 17, 1182 CrossRef.
- M. Antolini, A. Bozzoli, C. Ghiron, G. Kennedy, T. Rossi and A. Ursini, Bioorg. Med. Chem. Lett., 1999, 9, 1023 CrossRef CAS PubMed.
- V. H. V Bossche, G. Willemsens, W. Cools, P. Marichal and W. Lauwers, Biochem. Soc. Trans., 1983, 11, 665 CrossRef.
- L. Wang, K. W. Woods, Q. Li, K. J. Barr, R. W. McCroskey, S. M. Hannick, L. Gherke, R. B. Credo, Y. H. Hui, K. Marsh, R. warner, J. Y. Lee, N. Zielinsky-mozng, D. Frost, S. H. Rosenberg and H. L. Sham, J. Med. Chem., 2002, 45, 1697 CrossRef CAS PubMed.
- A. Puratchikody and M. Doble, Bioorg. Med. Chem., 2007, 15, 1083 CrossRef CAS PubMed.
- R. Schmierer, H. Mildenbergr and H. Buerstell, German Pat., 361464, 1987Chem. Abstr., 108, 37838, 1988.
- J. Freedman and J. Loscalzo, New Therapeutic Agent in Thrombosis and Thrombolysis, Taylor and Francis, New York, 3rd edn, 2009 Search PubMed.
- A. K. Takle, M. J. B. Brown, S. Davies, D. K. Dean, G. Francis, A. Gaiba, A. W. Hird, F. D. King, P. J. Lovell, A. Naylor, A. D. Reith, J. G. Steadman and D. M. Wilson, Bioorg. Med. Chem. Lett., 2006, 16, 378 CrossRef CAS PubMed.
- J. C. Lee, J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Keys, S. W. L. Vatter, J. E. Stricker, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, G. P. Livi, J. R. White, J. L. Adams and P. R. Young, Nature, 1994, 372, 739 CrossRef CAS PubMed.
- S. E. de Laszlo, C. Hacker, B. Li, D. Kim, M. MacCoss, N. Mantlo, J. V. Pivnichny, L. Colwell, G. E. Koch, M. A. Cascieri and W. K. Hagmann, Bioorg. Med. Chem. Lett., 1999, 9, 641 CrossRef CAS PubMed.
- (a) S. Balalaie, M. M. Hashemi and M. Akhbari, Tetrahedron Lett., 2003, 44, 1709 CrossRef CAS; (b) S. Samai, G. C. Nandi, P. Singh and M. S. Singh, Tetrahedron, 2009, 65, 10155 CrossRef CAS.
- (a) S. E. Wolkenberg, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef CAS PubMed; (b) S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539 CrossRef CAS.
- (a) M. V. Chary, N. C. Keerthysri, S. V. N. Vupallapati, V. N. Srinivasu, N. Lingaiah and S. Kantevari, Catal. Commun., 2008, 9, 2013 CrossRef CAS; (b) M. Kidwai, P. Mothsra, V. Bansal, R. K. Somvanshi, A. S. Ethayathulla, S. Dey and T. P. Singh, J. Mol. Catal. A: Chem., 2007, 265, 177 CrossRef CAS.
- (a) A. Teimouri and A. N. Chermahini, J. Mol. Catal. A: Chem., 2011, 346, 39 CrossRef CAS; (b) L. Nagarapu, S. Apuri and S. Kantevari, J. Mol. Catal. A: Chem., 2007, 266, 104 CrossRef CAS.
- (a) J. N. Sangshetti, N. D. Kokare, S. A. Kotharkara and D. B. Shinde, J. Chem. Sci., 2008, 5, 463 CrossRef; (b) A. R. Karimi, Z. Alimohammadi, J. Azizian, A. A. Mohammadi and M. R. Mohmmadizadeh, Catal. Commun., 2006, 7, 728 CrossRef CAS.
- (a) L. Nagarapu, S. Apuri and S. Kantevari, J. Mol. Catal. A: Chem., 2007, 266, 104 CrossRef CAS; (b) M. M. Heravi, F. Derikvand and M. Haghighi, Monatsh. Chem., 2008, 139, 31 CrossRef CAS.
- (a) A. Shaabani and A. Rahmati, J. Mol. Catal. A: Chem., 2006, 249, 246 CrossRef CAS; (b) M. M. Heravi, K. Bakhtiari, H. A. Oskooie and S. Taheri, J. Mol. Catal. A: Chem., 2007, 263, 279 CrossRef CAS; (c) G. V. M. Sharma, Y. Jyothi and P. S. Lakshmi, Synth. Commun., 2006, 36, 2991 CrossRef CAS.
- (a) Y. B. Kim, Y. H. Kim and J. Y. Park, Bioorg. Med. Chem. Lett., 2004, 14, 541 CrossRef CAS PubMed; (b) S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Chem. Commun., 2003, 18, 2286 RSC.
- (a) A. Jaso, B. Zarranz, I. Aldana and A. Monge, J. Med. Chem., 2005, 48, 2019 CrossRef CAS PubMed; (b) X. Hui, J. Desrivot, C. Bories, P. M. Loiseau, X. Franck, R. Hocquemiller and B. Figadere, Bioorg. Med. Chem. Lett., 2006, 16, 815 CrossRef CAS PubMed.
- (a) C. Venkatesh, B. Singh, P. K. Mahata and H. Junjappa, Org. Lett., 2005, 7, 2169 CrossRef CAS PubMed; (b) V. Jeena, S. Sithebe and R. S. Robinson, Synth. Commun., 2015, 45(12), 1484 CrossRef CAS; (c) M. Esmaeilpour and A. R. Sardarian, Green Chem. Lett. Rev., 2014, 7(3), 301 CrossRef CAS.
- (a) S. V. More, M. N. V. Sastry and C. F. Yao, Green Chem., 2006, 8, 91 RSC; (b) H. Tai Kun and W. Rui, Catal. Commun., 2008, 9, 1143 CrossRef.
- (a) L. H. Sternbach, Angew. Chem., Int. Ed. Engl., 1971, 10, 34 CrossRef CAS PubMed; (b) J. R. De Baun, F. M. Pallos and D. R. Baker, US Pat., 3, 978, 227, 1976Chem. Abstr., 1977, 86, 5498d.
- (a) L. O. Randall, B. Kappel, S. Garattini, E. Mussini and L. O. Randall, Benzodiazepines, Raven Press, New York, 1973, p. 27 Search PubMed; (b) R. I. Fryer, Bicyclic Diazepines, in Comprehensive Heterocyclic Chemistry, ed. E. C. Taylor, Wiley, New York, NY, USA, 1991, vol. 50, ch. II Search PubMed.
- (a) D. Jung, J. Song, Y. Kim, D. Lee, Y. Lee, Y. Park, S. Choi and J. Hahn, Bull. Korean Chem. Soc., 2007, 28(10), 1877 CrossRef CAS; (b) M. Munoz, G. Sathicq, G. Romanelli, S. Hernandez, C. I. Cabello, I. L. Botto and M. Capron, J. Porous Mater., 2013, 20, 65 CrossRef CAS; (c) K. Rachedine, B. Norah, B. Saliha, B. Sihame, R. Cherifa and N. K. Bellara, Molecules, 2011, 16, 92 Search PubMed.
- (a) M. Pozarentzi, J. S. Stephanatou and C. A. Tsoleridis, Tetrahedron, 2002, 43, 1755 CrossRef CAS; (b) P. Hazarika, P. Gogoi, S. Hatibaruah and D. Konwar, Green Chem. Lett. Rev., 2011, 4, 327 CrossRef CAS; (c) G. D. Yadav and A. R. Yadav, Ind. Eng. Chem. Res., 2013, 52(50), 17812 CrossRef CAS.
- K. C. Patil, M. S. Hegde, T. Rattan and S. T. Aruna, Chemistry of Nano Crystalline Oxide Materials, Combustion Synthesis, Properties and Applications, World Science publishing Pvt. Ltd, Singapore, 2008 Search PubMed.
- J. D. Angel, A. F. Aguilera, I. R. Galindo, M. Martinez and T. Viveros, Mater. Sci. Appl., 2012, 3, 650 Search PubMed.
- J. O. Landeros, M. E. Contreras and H. Pfeiffer, J. Mater. Chem. C, 2009, 16(4), 473 Search PubMed.
- V. Santos, M. Zeni, C. P. Bergmann and J. M. Hohemberger, Rev. Adv. Mater. Sci., 2008, 17(1), 62 CAS.
- D. H. Maria, R. A. Ana, A. M. Jacob and M. Guido, Catal. Today, 2009, 143, 326 CrossRef.
- J. Chandradass, B. Jun and D. S. Bae, J. Non-Cryst. Solids, 2008, 354, 3085 CrossRef CAS.
- J. R. Sohn, E. W. Chun and Y. I. Pae, Bull. Korean Chem. Soc., 2003, 24, 1785 CrossRef CAS.
- S. Yu, P. Jiang, Y. Dong, P. Zhang, Y. Zhang and W. Zhang, Bull. Korean Chem. Soc., 2012, 33(2), 524 CrossRef CAS.
- J. Safari, Z. Akbari and S. Naseh, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.10.012.
- N. Thimmaraju, S. Z. Mohamed Shamshuddin, S. R. Pratap and K. Shyam Prasad, Arabian J. Chem., 2014 DOI:10.1016/j.arabjc.2014.12.011.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13956f |
| This journal is © The Royal Society of Chemistry 2016 |