Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
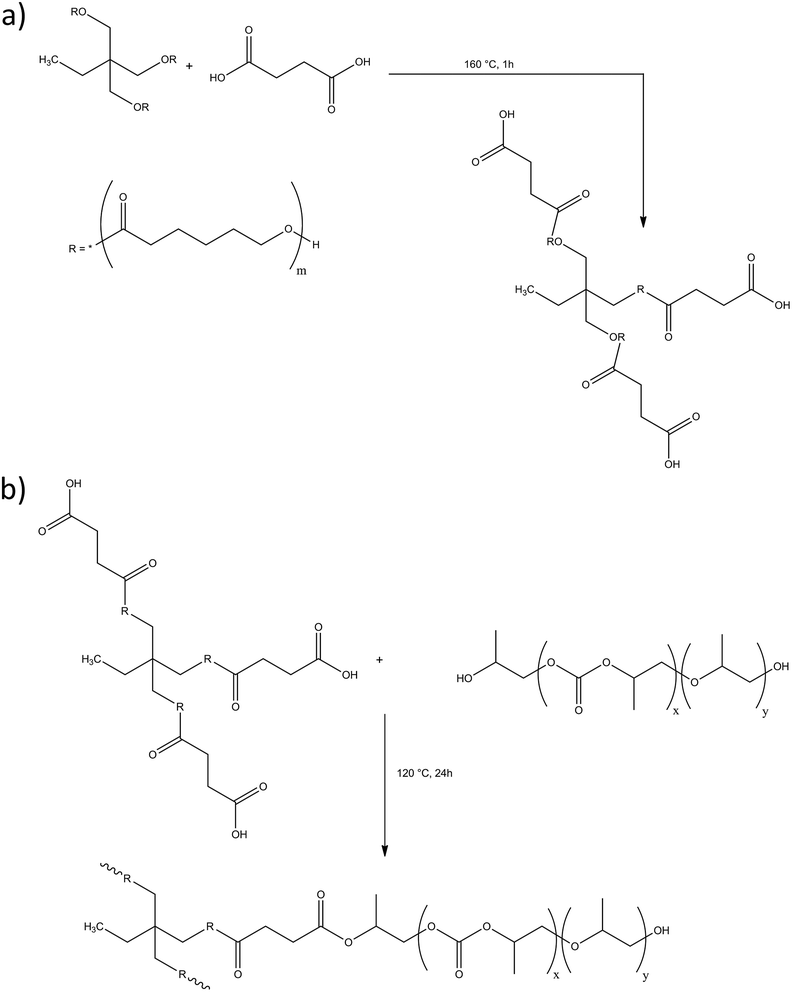
One-pot, mouldable, thermoplastic resins from poly(propylene carbonate) and poly(caprolactone triol)†
S. Spoljaric and
J. Seppälä*
Polymer Technology Research Group, Department of Biotechnology and Chemical Technology, School of Chemical Technology, Aalto University, P.O Box 16100, Aalto, Finland. E-mail: jukka.seppala@aalto.fi
First published on 1st April 2016
Abstract
Co-polymers of poly(propylene carbonate) (PPC) and poly(caprolactone triol) (PCLT) were synthesised via a simple yet effective one-pot, two-step method, without the need for a catalyst or solvent. Successful synthesis was confirmed via 1H NMR and ATR-FT-IR. The co-polymers displayed increased thermal stability. Covalent bonding between the functionalised PCLT and PPC enhanced moduli and strength, while also reducing creep and flow within PPC. Co-polymers displayed increased storage moduli, glass transition temperatures and damping properties. Additionally, PCLT–PPC co-polymers were flexible and easily moulded into shapes while maintaining their form and structural integrity. By imparting a relatively small concentration of functionalised-PCLT, the existing material properties (damping), as well as those previously seen as a hindrance to greater PPC application (poor thermal stability, cold flow, poor strength) were enhanced, while new characteristics (mouldability) were introduced.
Introduction
Synthetic polymers, mostly prepared from non-renewable fossil fuels, have been a staple material in many applications and industries for well over a century. However, the rapid consumption of petroleum, coupled with factors such as pollution and growing environmental consciousness have shifted the research focus towards the development of biodegradable polymers from renewable feedstocks.1,2 One such polymer is poly(propylene carbonate) (PPC), a thermoplastic aliphatic polyester which is prepared via copolymerisation of carbon dioxide (CO2) and propylene oxide.3 Apart from providing a value-added pathway of utilising CO2, PPC's other favourable attributes include biodegradability,4 renewability, cost effectiveness,5 transparency6 and exceptional barrier7 and elastic/ductile properties. Despite its mass appeal and the recent reporting of large-scale production capabilities,8 its greater applicability is limited by its poor mechanical strength and low glass transition temperature Tg of ∼40 °C,9 often leading to cold flow under ambient conditions.10 Thus, imparting strength, rigidity and structural integrity into PPC is a major consideration when further applicability and use is concerned.The introduction of branching or crosslinking (facilitated via blending or co-polymerisation with compatible polymers) is a convenient and efficient approach to overcoming PPC's hindrances. In particular, the introduction of branched structures is preferred in order to retain the thermoplastic nature of PPC. One of the most promising candidates to fulfil this role is poly(caprolactone triol) (PCLT), having been successfully utilised previously to toughen poly(lactic acid)11,12 and as a component in various polyurethane systems.13,14 Furthermore, PCLT is biodegradable,15 biocompatible,16 miscible with a wide range of polymers and displays superior UV resistance properties. Although poly(caprolactone) (PCL) is synthesised from petroleum-derived sources,17 potential exists to prepare PCL precursors from renewable sources, such as glucose and D-fructose.18,19 This drive towards utilising polymers from renewable resources has produced limited research regarding the preparation of PPC–PCL blends and co-polymers,20 exploring possible applications including nanofibrilar scaffolds21 and toughening agents for epoxy resins.22 However, the aforementioned compositions utilise linear poly(ε-caprolactone) rather than the triol form, providing opportunity to probe the reinforcing ability of PCLT. Likewise, the mechanical and rheological data regarding PPC–PCLT co-polymers is rather limited; this provides great potential in exploring possible alternative applications, especially with regard to films.
Herein, the synthesis and characterisation of PPC–PCLT co-polymers is described. Co-polymer synthesis consisted of a one-pot, two-step approach; firstly, a polycondensation reaction between PCLT and either succinic or sebacic acid introduced carboxylic acid moieties to the chain ends. The second step involved an esterification reaction between the carboxylated PCLT pre-polymer and hydroxyl end groups of PPC. The co-polymer synthesis proved to be a simple yet effective method, requiring no solvent or catalyst. To the best of the authors' knowledge, this is the first reported synthesis of such co-polymers. The mechanical, rheological, thermal and morphological properties of the co-polymers were characterised.
Experimental
Materials
Poly(propylene carbonate) (PPC, Mw ∼ 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, cyclic propylene carbonate content 4.0–5.5 mol%), poly(caprolactone triol) (PCLT, Mw ∼ 900), succinic acid (≥99.0%) and sebacic acid (99%) were purchased from Sigma Aldrich, USA and used as received.
000, cyclic propylene carbonate content 4.0–5.5 mol%), poly(caprolactone triol) (PCLT, Mw ∼ 900), succinic acid (≥99.0%) and sebacic acid (99%) were purchased from Sigma Aldrich, USA and used as received.
Synthesis of PPC–PCLT co-polymers
The synthesis of the PPC–PCLT co-polymers (using succinic acid as an example) is summarised in Scheme 1. PCLT and either succinic- or sebacic acid were charged into a twin-neck 100 mL round-bottom flask. The molar ratio between PCLT and succinic/sebacic acid was either 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The contents of the flask were stirred at 160 °C for 1 h, after which the pre-polymer mixture was cooled to 120 °C. PPC was added to the flask (PCLT
3. The contents of the flask were stirred at 160 °C for 1 h, after which the pre-polymer mixture was cooled to 120 °C. PPC was added to the flask (PCLT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPC molar ratio of 1
PPC molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and the reaction mixture was stirred for 24 h. Films for subsequent use and analysis were prepared using a heated press (TP400 Table Press, Fortune Holland, Netherlands). Films were heated at 100 °C, held under 150 kPa of pressure for 5 min then slowly cooled to ambient. The nomenclature and composition of the PPC–PCLT-copolymers is summarised in Table 1.
3) and the reaction mixture was stirred for 24 h. Films for subsequent use and analysis were prepared using a heated press (TP400 Table Press, Fortune Holland, Netherlands). Films were heated at 100 °C, held under 150 kPa of pressure for 5 min then slowly cooled to ambient. The nomenclature and composition of the PPC–PCLT-copolymers is summarised in Table 1.
| Sample name | Dicarboxylic acid | PCLT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dicarboxylic acid ratio (mol) dicarboxylic acid ratio (mol) |
PCLT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPC ratio (mol) PPC ratio (mol) |
|---|---|---|---|
| CP-Seb1 | Sebacic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| CP-Seb3 | Sebacic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| CP-Suc1 | Succinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| CP-Suc3 | Succinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Characterisation of PPC–PCLT co-polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) were measured as a function of temperature from −100 to 120 °C at a heating rate of 3 °C min−1.
δ) were measured as a function of temperature from −100 to 120 °C at a heating rate of 3 °C min−1.Results and discussion
Co-polymer structure and chemistry
| Sample name | Mw (g mol−1) | Mn (g mol−1) | Mw/Mn |
|---|---|---|---|
| PPC | 156![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 116 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 114 |
3.54 |
| PCLT | 2091 | 1620 | 1.29 |
| CP-Seb1 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193 193 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
4.19 |
| CP-Seb3 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537 537 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588 588 |
2.90 |
| CP-Suc1 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704 704 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 143 |
3.84 |
| CP-Suc3 | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 906 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
3.47 |
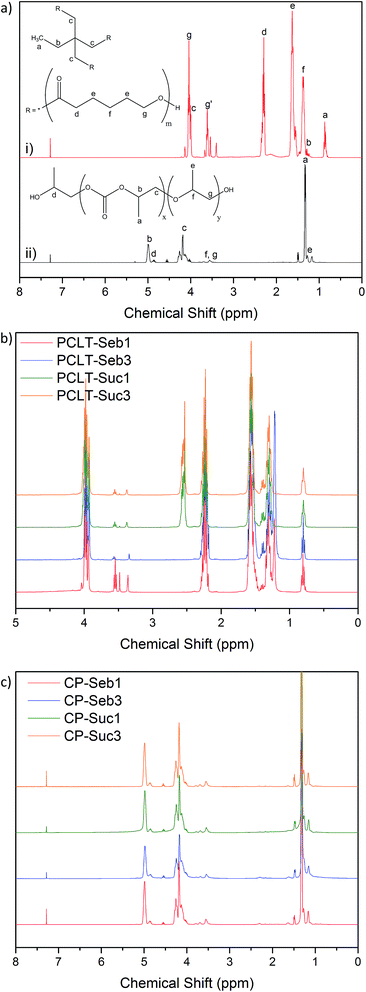 | ||
| Fig. 1 1H NMR spectra; (a) pure polymers (i) PCLT, (ii) PPC, (b) PCLT-diacid pre-polymers, (c) PPC–PCLT co-polymers. | ||
Peaks a through c correspond to various protons from within the core of PCLT, while peaks d through g are attributed to the various protons within the repeating units. In particular, the peaks g′ and g were assigned to –CH2– protons next to terminal hydroxyl end groups and esterified –OH linkages, respectively.31 These esterified hydroxyls can be linked to a following repeating unit or, as in this case, covalently bonded with either succinic or sebacic acid. Subsequently, the integrals of peaks g and g′ were compared to determine the extent of hydroxyl conversion and subsequently confirm the successful reaction between PCLT and succinic/sebacic acid; these results are summarised in Table S1 in the ESI material,† while the spectra of the PCLT-diacid pre-polymers are presented in Fig. 1b. Pure PCLT has a –OH content of 27.0%; this value reduced to 15.2 and 12.0% following the reaction with 1 mole of sebacic or succinic acid, respectively. As anticipated, the lowest values were obtained following reactions with 3 moles of succinic or sebacic acid (2.4 and 1.9%, respectively). Furthermore, the co-polymer spectra displayed novel peaks corresponding to the specific diacid; PCLT-Seb1 and PCLT-Seb3 exhibited a peak at 1.2 ppm, corresponding to the methyl protons of sebacic acid, while PCLT-Suc1 and PCLT-Suc3 displayed a peak at 2.5 ppm, attributed to the methyl groups within the centre of the succinic acid chain. Likewise, these peak intensities increased with molar ratio. This data confirms successful esterification between the PCLT and diacids, with varying degrees of conversion dependent on the concentration of diacid utilised.
The spectra of the PPC–PCLT co-polymers are displayed in Fig. 1c. The structure of PPC dominates the spectra of all co-polymers, due to its greater concentration within the compositions. However, signals corresponding to the PCLT-diacid copolymers can be noticed to a certain degree (e.g. the trace signal at ∼2.2 ppm). As with the pre-polymers, peak integral of the PPC–PCLT co-polymer spectra were compared to determine the extent of esterification between the PPC and PCLT-diacid pre-polymers. Peaks b (5 ppm) and d (4.9 ppm) from the PPC spectra (refer Fig. 1a) were assigned to –CH protons adjacent to either the carbonate ester within the repeating unit or terminal hydroxyl groups, respectively. Neither PCLT, succinic acid nor sebacic acid display any overlapping peaks within this region. The co-polymer peak integrals and percentage –OH are summarised in Table S2 in the ESI material.† The percentage of –OH end groups within pure PPC was 11.8%; this dropped to 9.0 and 8.9% for CP-Seb1 and CP-Suc1, respectively. Thus, the proportion of –OH groups within PPC reduced within the co-polymers, indicating successful covalent bonding with the PCLT-diacid pre-polymer. As anticipated, increasing the degree of substitution within the pre-polymers ultimately provides more sites for esterification within the co-polymers; as a result the lowest –OH percentages were recorded for CP-Seb3 (8.6%) and CP-Suc3 (8.0%). The NMR data confirmed the successful synthesis of both the pre- and co-polymer, while also highlighting the influence of PCLT-diacid degree of substitution on subsequent PPC–PCLT covalent bonding.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dicarboxylic acid ratio of 1
dicarboxylic acid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, due to the increased concentration of sebacic- or succinic acid. This behaviour strongly indicates the successful esterification between PCLT and sebacic- or succinic acid, thereby confirming successful pre-polymer formation.
3, due to the increased concentration of sebacic- or succinic acid. This behaviour strongly indicates the successful esterification between PCLT and sebacic- or succinic acid, thereby confirming successful pre-polymer formation.
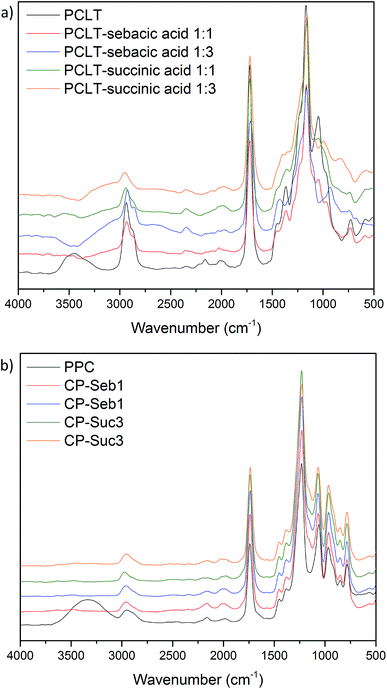 | ||
| Fig. 2 FT-IR spectra; (a) of pure PCLT and PCLT-dicarboxylic acid pre-polymers, (b) pure PPC and PPC–PCLT co-polymers. | ||
The IR spectra of pure PPC and the PPC–PCLT co-polymers are presented in Fig. 2b. All co-polymers displayed spectra with similar peaks and bands, indicative of both PCLT and PPC structure; 2951 cm−1 (C–H stretching), 1736 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1229 cm−1 (O–C–O stretching) and 1070 cm−1 (C–O stretching).33,34 However, unlike pure PPC, no distinct peak is noticed at 3338 cm−1 (O–H stretching and indicative of the presence of hydroxyl end groups on PPC). This behaviour is in correlation with the 1H NMR data and confirms that PCLT-dicarboxylic acid pre-polymers and PPC successfully reacted to form PPC–PCLT co-polymers.
O stretching), 1229 cm−1 (O–C–O stretching) and 1070 cm−1 (C–O stretching).33,34 However, unlike pure PPC, no distinct peak is noticed at 3338 cm−1 (O–H stretching and indicative of the presence of hydroxyl end groups on PPC). This behaviour is in correlation with the 1H NMR data and confirms that PCLT-dicarboxylic acid pre-polymers and PPC successfully reacted to form PPC–PCLT co-polymers.
Thermal stability and degradation
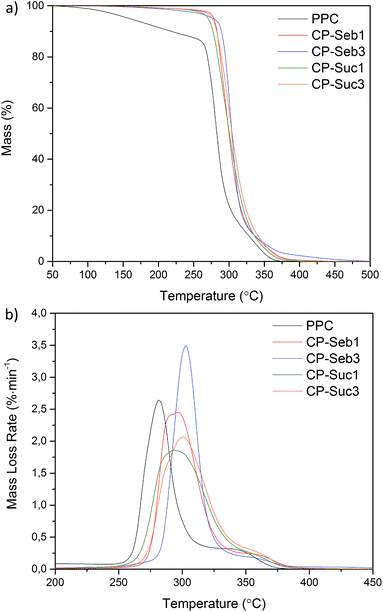
The mass loss curves of pure PPC and the PPC–PCLT co-polymers are presented in Fig. 3a. The degradation of pure PPC began with a chain unzipping step, attributed to a ‘backbiting’ attack of the terminal hydroxyl groups on the carbonyl group.35 This continues until ∼256 °C where the main mass-loss event corresponding to random chain scission takes place.36 All PPC–PCLT displayed a noticeable reduction in mass loss during chain unzipping. As mentioned previously, the unzipping mechanism is dependent on the availability of hydroxyl end groups likely to attack carbonyl groups. Ester linkage formation between the functionalised PCLT and PPC reduces the total number hydroxyls able to partake in chain unzipping, essentially stabilising the polymer. Furthermore, all PPC–PCLT co-polymers underwent chain scission at higher temperatures than pure PPC, as indicated by the curves shifting towards higher temperatures. The introduction of covalent bonds via the terminal hydroxyls of PPC and carboxylic acid end groups of diacid-functionalised PCLT imparts restrictions on the mobility of polymer chain segments. As a result, the amount of energy required for rotation about bonds and subsequent chain scission is increased, leading to increased thermal stability.In order to further probe the degradation behaviour, as well as characterise the influence of diacid concentration and type, the derivative mass loss curves were taken; these are presented in Fig. 3b. The temperature at which the maximum rate of degradation (Td) occurred for PPC was 281 °C; this increased to 293 and 296 °C for co-polymer CP-Seb1 and CP-Suc1, respectively. Maximum Td values were observed for CP-Seb3 (303 °C) and CP-Suc3 (300 °C). This corresponds with the aforementioned relationship between increased functionalisation of PCLT leading to increased covalent bonding with PPC and, subsequently, superior thermal stability. When considering the influence of diacid structure, co-polymers containing sebacic acid-functionalised PCLT displayed increased Td and narrower curves than their succinic acid counterparts. This may be attributed to the boiling point of sebacic acid (294 °C) being higher than that of succinic acid (235 °C), allowing co-polymers containing sebacic acid to display enhanced thermal stability at elevated temperatures. The TGA data highlights the enhanced thermal stability of the PPC–PCLT co-polymers compared with pure PPC, potentially broadening the processability and utilisation of PPC-based polymers, which have been somewhat limited due to poor thermal properties.
Mechanical properties
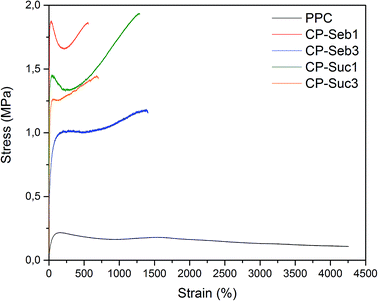
The stress–strain curves of the PPC–PCLT co-polymers were in significant contrast to that of PPC; a drastic increase in modulus, yield strength and tensile strength were observed within all co-polymers. CP-Seb1 displayed the most pronounced enhancements in modulus (58.3 MPa), yield strength (1.88 MPa) and tensile strength (1.85 MPa). These values were 62.7, 8.5 and 8.4 times higher than the respective modulus, yield strength and tensile strength of pure PPC. This is indicative of ‘reinforcing’ influence of PCLT on PPC; covalent bonding between PCLT and PPC introduces branching into the linear PPC chain, which restrict polymer chain motions and sliding during periods of applied load. Subsequently, strength and rigidity are enhanced at the expense of elongation. Consequently, the elongation at break of the co-polymers was considerably reduced compared to pure PPC. As indicated in the 1H NMR data, a small degree of unreacted sebacic- or succinic acid remained in the co-polymers. Salgado et al.37 prepared blended PCL (diol)–sebacic acid gels which were rigid and solid in nature. Thus, residual diacid could potentially contribute towards the increase in strength and stiffness. However, given the relatively high degree of conversion during PCLT functionalisation, the influence of unbound diacid can be considered minor.
When comparing modulus values, the ratio of PCLT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dicarboxylic acid utilised during pre-polymer synthesis had a distinct influence on the tensile behaviour of the co-polymers. The moduli values of CP-Seb1 (58.3 MPa) and CP-Suc1 (55.8 MPa) were markedly higher than those of CP-Seb3 (23.8 MPa) and CP-Suc3 (28.3 MPa). Furthermore, the tensile and yield strength of CP-Seb1 and CP-Suc1 were greater than their 3 mole pre-polymer counterparts (CP-Seb3 and CP-Suc3, respectively). Within co-polymers containing PCLT reacted with 3 moles of diacid, the degree of covalent linkages with PPC is increased, as confirmed with NMR. This excessive bonding may impart brittleness into the co-polymers. In contrast, co-polymers containing PCLT functionalised with 1 mole of diacid allow reinforcement to be imparted without excessive restrictions on polymer chain segment motions.
dicarboxylic acid utilised during pre-polymer synthesis had a distinct influence on the tensile behaviour of the co-polymers. The moduli values of CP-Seb1 (58.3 MPa) and CP-Suc1 (55.8 MPa) were markedly higher than those of CP-Seb3 (23.8 MPa) and CP-Suc3 (28.3 MPa). Furthermore, the tensile and yield strength of CP-Seb1 and CP-Suc1 were greater than their 3 mole pre-polymer counterparts (CP-Seb3 and CP-Suc3, respectively). Within co-polymers containing PCLT reacted with 3 moles of diacid, the degree of covalent linkages with PPC is increased, as confirmed with NMR. This excessive bonding may impart brittleness into the co-polymers. In contrast, co-polymers containing PCLT functionalised with 1 mole of diacid allow reinforcement to be imparted without excessive restrictions on polymer chain segment motions.
All co-polymers experienced strain hardening following yielding. Within amorphous polymers (such as PPC), strain hardening believed to result from segments between entanglements being pulled taut during load application; this leads to an increase in free energy due to reduced configurational entropy.38 Through the addition of functionalised PCLT, entanglements are introduced between PPC chain segments. Subsequently, the strain hardening behaviour of the co-polymers is more pronounced than pure PPC. Sub-Tg annealing can also enhance the effects of strain hardening, due to reduction in free volume and internal energy changes.39 Table S4 in the ESI† displays the glass transition (Tg) values of pure PPC and the co-polymers obtained via DMA. The Tg of all polymers ranged from 37–46 °C, while the storage and testing temperature was 23 °C. Thus, pure PPC and the co-polymers were effectively annealed, which can contribute to the strain hardening behaviour. It is worth noting that CP-Seb1 and CP-Seb3 had the lowest Tg values, 42 and 41 °C respectively. Due to the close proximity of the Tg and testing temperature, limited viscous flow may also partly contribute to the high degree of strain hardening exhibited by these two polymers.
As shown in Fig. 4, CP-Seb1 exhibited significantly higher yield strength and tensile strength values than CP-Seb3, while the contrast between CP-Suc1 and CP-Suc3 was less drastic. This difference in magnitude of tensile properties may be attributed to the influence of diacid chain length. The incorporation of succinic acid introduces a 4-carbon chain into the polymer structure, which may have negligible difference on material properties due to the small size. In contrast, sebacic acid contains a significantly-longer 10-carbon chain. This increase in chain length promotes chain entanglement and secondary interactions between polymer segments, leading to enhanced strength. Although CP-Seb3 displayed the lowest tensile- and yield strength values, this was attributed to brittleness derived from excessive covalent bonding. The tensile data indicates that co-polymer modulus (stiffness) and yielding behaviour is primarily influenced by the degree of covalent bonding, while strength is dependent on the length of the diacid used during pre-polymer synthesis.
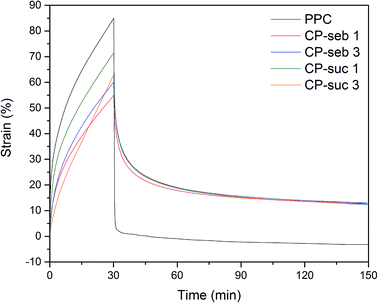
As the functionalisation of the PCLT pre-polymer increased from 1 mole to 3 moles of succinic or sebacic acid, creep reduced while permanent deformation increased. This was anticipated, as the greater number of carboxylic acid groups imparted onto PCLT increases the number of sites available for potential esterification with PPC. Subsequently, a greater degree of covalent bonding leads to more restrictions on chain motion. Conversely, co-polymers functionalised with sebacic acid experienced less creep and more permanent deformation than their succinic acid-functionalised counterparts. One proposed mechanism stems from the difference in diacid chain length on the PCLT pre-polymer; the longer, 10-carbon chain of sebacic acid may encourage chain entanglement and secondary interactions amongst PPC, leading to increased hindrance on segmental motion. In contrast, such aforementioned phenomena may be less likely with shorter, 4-carbon chain succinic acid.
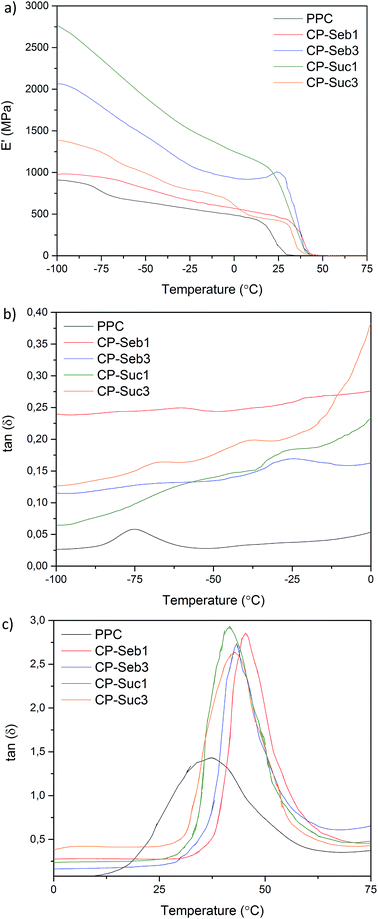 | ||
| Fig. 6 DMA curves of pure PPC and PPC–PCLT co-polymers; (a) storage moduli, (b) tan(δ) within the sub-Tg (PPC) region, (c) tan(δ) glass transition region of PPC. | ||
All co-polymers displayed increased E′ values relative to pure PPC, indicative of increased rigidity and stiffness within the co-polymers. Furthermore, PCLT has a Tm of ∼6 °C; crystalline domains may contribute towards the storage modulus at upon cooling below Tm. For specimens CP-Seb1 and CP-Seb3, increasing the larger E′ value of the latter is attributed to the greater degree of substitution on the functionalised PCLT precursor. Due to the increased number of reactive sites on PCLT able to covalently bond with PPC, the diacid-functionalised PCLT can impart a greater degree of restrictions/hindrances on PPC-chain motion. Furthermore, the increased length of sebacic acid segments may encourage entanglement formation, leading to further chain restrictions and subsequent enhanced stiffness. This can account for CP-Seb3 displaying a higher E′ than CP-Suc3.
CP-Suc1 displayed the highest E′ values, while CP-Suc3 displayed a reduction in storage modulus; the behaviour being opposite to that displayed by co-polymers CP-Seb1 and CP-Seb3. It is worth noting that the tensile moduli of CP-Suc1 obtained during stress–strain analysis was similar to that of CP-Seb1 (refer to Table S3 in the ESI material†). This behaviour may be attributed to the shorter succinic acid chains imparting a brittling influence at higher degrees of substitution (as is the case with CP-Suc3). However, at lower degrees of substitution (as with CP-Suc1), successful reinforcement may be attainable without excessive restriction on PPC chain segment motion.
The maximum from the tan(δ) curves was used to indicate the Tg; these values and other thermal transitions are summarised in Table S4 in the ESI.† Pure PPC displayed a Tg of 37 °C; this increased for all co-polymers, with CP-Seb1 yielding the highest Tg of 46 °C. At temperatures below the Tg peak, co-polymer spectra displayed increased tan(δ) heights relative to pure PPC. These observations are indicative of increased restrictions on segmental motion of PPC; via covalent bonding and physical entanglements.42 Co-polymers prepared with sebacic acid displayed higher Tg values than their succinic acid counterparts. This is attributed to the increased chain length of sebacic acid; the probability of chain entanglement formation increases with chain length, resulting in a greater degree of restrictions being imparted on PPC chain motion.
Co-polymers showed a significant increase in tan(δ) peak intensity, indicative of enhanced damping properties. The damping ability of a polymer is believed to be influenced by two factors; (a) the amount of free volume, (b) internal friction between macromolecules across the glass transition region.43 Considering these two criteria, two possible explanations for the enhanced damping properties are proposed. Firstly, covalent bonding between the three-armed functionalised PCLT and linear PPC introduces a three-dimensional network structure to the system. This may be compared with branching44 or crosslinking45 which can entrap free volume within the system (while also enhancing stiffness, as observed in the E′ curves). Secondly, there may be weak interfacial interaction between entangled segments of PCLT and PPC. This poor interaction is especially vulnerable when subjected to external stresses or if both polymers are in the glassy state46 (PPC is amorphous, while the Tg of PPC is greater than the Tm of PCLT). Subsequently, PPC chain motions at the interface may slip more easily, leading to increased free volume and damping ability.47
Conclusions
Co-polymers of PCLT functionalised with either succinic- or sebacic acid and PPC were functionalised utilising a one-pot, two step method. 1H NMR and ATR-FT-IR confirmed the structure and successful synthesis of the co-polymers. Co-polymers displayed enhanced thermal stability compared with pure PPC. This was attributed to the esterification reaction between PPC and diacid-functionalised PCLT (a) reducing the number of vacant hydroxyl groups able to participate in chain unzipping, and (b) restricting segmental motion of PPC chain segments. This dramatically broadens the processability and subsequent utilisation potential of PPC. All co-polymers exhibited superior tensile moduli, strength, storage moduli, glass transition temperatures and damping ability than pure PPC, while also displaying reduced creep and flow properties. The ability of the functionalised PCLT to influence PPC rheological and cold-flow properties was highlighted by the PPC–PCLT co-polymers being readily mouldable and maintaining a greater degree of dimensional stability compared with pure PPC.Two factors primarily governed the mechanical properties. Firstly, increasing the degree of substitution of the functionalised PCLT increased the likelihood of ester-linkage formation with PPC. This resulted in greater restrictions on PPC chain motions, reflected in increased rigidity and yielding behaviour. Secondly, functionalising PCLT with the longer sebacic acid increases the likelihood of entanglement formation, leading to further restrictions on PPC chain motion, resulting in enhanced strength properties. These restrictions on chain motion (in the form of ester bonds and entanglements) were evident when load was applied and removed, as demonstrated by the reduced creep behaviour and dimensional stability following hand-moulding, respectively. The enhanced damping properties were attributed to pockets of free volume trapped within the co-polymer, and reduced internal friction between PCLT and PPC.
The drastic enhancement in thermal stability, tensile behaviour, creep/flow properties and damping ability at relatively low PCLT concentrations provides great potential for further expanding the potential applications of PPC and its blends/co-polymers. Namely, consumer products, packaging, construction and biomedical applications are amongst the most promising application fields worth pursuing.
Notes and references
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- L. Montero de Espinosa and M. A. R. Meier, Eur. Polym. J., 2011, 47, 837–852 CrossRef CAS.
- G. Kim, M. Ree, H. Kim, I. Kim, J. Kim and J. Lee, Macromol. Res., 2008, 16, 473–480 CrossRef CAS.
- X. C. Ge, X. H. Li, Q. Zhu, L. Li and Y. Z. Meng, Polym. Eng. Sci., 2004, 44, 2134–2140 CAS.
- X. C. Ge, Y. Xu, Y. Z. Meng and R. K. Y. Li, Compos. Sci. Technol., 2005, 65, 2219–2225 CrossRef CAS.
- X. Jing, M. R. Salick, T. Cordie, H. Mi, X. Peng and L. Turng, Ind. Eng. Chem. Res., 2014, 53, 9391–9400 CrossRef CAS.
- G. Flodberg, I. Helland, L. Thomsson and S. Bodil Fredriksen, Eur. Polym. J., 2015, 63, 217–226 CrossRef CAS.
- L. Huihong, P. Lisha, L. Qiang, X. Nai, L. Lingbin, P. Sujuan and F. Songbao, e-Polym., 2010, 038, 1–9 Search PubMed.
- X. Wu, H. Zhao, B. Nörnberg, P. Theato and G. A. Luinstra, Macromolecules, 2014, 47, 492–497 CrossRef CAS.
- G. Luinstra and E. Borchardt, Adv. Polym. Sci., 2012, 245, 29–48 CrossRef CAS.
- D. V. Mistura, A. D. Messias, E. A. R. Duek and M. A. T. Duarte, Artif. Organs, 2013, 37, 978–984 CrossRef CAS PubMed.
- M. Duarte, A. Motta and E. Duek, Int. J. Polym. Sci., 2014, 2014, 1–9 CrossRef.
- Z. Li, Z. Zhang, K. L. Liu, X. Ni and J. Li, Biomacromolecules, 2012, 13, 3977–3989 CrossRef CAS PubMed.
- T. Wang, F. Huang and S. Lee, Polym. Int., 2002, 51, 1348–1352 CrossRef CAS.
- K. Lee, D. Kim and B. Kim, Biotechnol. Bioprocess Eng., 2007, 12, 152–156 CrossRef CAS.
- N. Castro, P. Goldstein and M. Cooke, Adv. Biosci. Biotechnol., 2011, 2, 167–173 CrossRef CAS.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- H. Hatakeyama, Y. Izuta, K. Kobashigawa, S. Hirose and T. Hatakeyama, Macromol. Symp., 1998, 130, 127–138 CrossRef CAS.
- T. Buntara, S. Noel, P. H. Phua, I. Melián-Cabrera, J. G. deVries and H. J. Heeres, Angew. Chem., Int. Ed., 2011, 50, 7083–7087 CrossRef CAS PubMed.
- Y. Hwang, M. Ree and H. Kim, Catal. Today, 2006, 115, 288–294 CrossRef CAS.
- G. Guo, Y. Wang, J. Deng, R. Fan, L. Zhou, A. Tong, Y. Zheng, X. Jianguo and X. Zhang, RSC Adv., 2015, 5, 82834–82844 RSC.
- S. Chen, B. Chen, J. Fan and J. Feng, ACS Sustainable Chem. Eng., 2015, 3, 2077–2083 CrossRef CAS.
- P. Song, Y. Shang, S. Chong, X. Zhu, H. Xu and Y. Xiong, RSC Adv., 2015, 5, 32092–32095 RSC.
- M. Yao, H. Deng, F. Mai, K. Wang, Q. Zhang, F. Chen and Q. Fu, eXPRESS Polym. Lett., 2011, 5, 937–949 CrossRef CAS.
- H. S. Shirazi, P. K. Forooshani, B. Pingguan-Murphy and I. Djordjevic, Prog. Org. Coat., 2014, 77, 821–829 CrossRef CAS.
- G. Shi, D. G. Cooper and M. Maric, Polym. Degrad. Stab., 2011, 96, 1639–1647 CrossRef CAS.
- J. Hilf, P. Schulze, J. Seiwert and H. Frey, Macromol. Rapid Commun., 2014, 35, 198–203 CrossRef CAS PubMed.
- A. Yoshida, S. Honda, H. Goto and H. Sugimoto, Polym. Chem., 2014, 5, 1883–1890 RSC.
- S. Wang, Y. Huang, B. Liao, G. Lin, G. Cong and L. Chen, Int. J. Polym. Anal. Charact., 1997, 3, 131–143 CrossRef CAS.
- W. Zhang, L. Lu, Y. Cheng, N. Xu, L. Pan, Q. Lin and Y. Wang, Green Chem., 2011, 13, 2701–2703 RSC.
- L. Xue, S. Dai and Z. Li, Macromolecules, 2009, 42, 964–972 CrossRef CAS.
- K. Muhammad, W. Abas, K. KimI, B. Pingguan-Murphy, N. Zain and H. Akram, Clinics, 2012, 67, 629–638 CrossRef PubMed.
- B. Fei, C. Chen, S. Peng, X. Zhao, X. Wang and L. Dong, Polym. Int., 2004, 53, 2092–2098 CrossRef CAS.
- L. A. Kanis, E. L. Marques, K. M. Zepon, J. R. Pereira, S. Pamato, M. T. de Oliveira, L. G. Danielski and F. C. Petronilho, J. Biomater. Appl., 2014, 29, 654–661 CrossRef CAS PubMed.
- J. An, Y. Ke, X. Cao, Y. Ma and F. Wang, Polym. Chem., 2014, 5, 4245–4250 RSC.
- T. J. Spencer and P. A. Kohl, Polym. Degrad. Stab., 2011, 96, 686–702 CrossRef CAS.
- C. L. Salgado, E. M. S. Sanchez, C. A. C. Zavaglia and P. L. Granja, J. Biomed. Mater. Res., Part A, 2012, 100, 243–251 CrossRef PubMed.
- V. S. Jatin and S. Basu, Int. J. Plast., 2014, 56, 139–155 CrossRef CAS.
- R. N. Haward, Macromolecules, 1993, 26, 5860–5869 CrossRef CAS.
- Y. Tao, X. Wang, X. Zhao, J. Li and F. Wang, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5329–5336 CrossRef CAS.
- J. Fried, Polymer Science and Technology, Prentice Hall PTR, 2nd edn, Upper Saddle River, 2003 Search PubMed.
- S. Han and L. Drzal, Curing Characteristics and Water Absorption behaviour of Glucose Based Polymers, in Frontiers in Polymer Research, ed. R. Bregg, Nova Sciences Publishers Inc, New York, 2006, pp. 1–26 Search PubMed.
- T. Trakulsujaritchok and D. J. Hourston, Eur. Polym. J., 2006, 42, 2968–2976 CrossRef CAS.
- D. Ladin, C. Park, S. Park and H. Naguib, SPE ANTEC Technical Papers. 46, 2000, vol. 2, pp. 1955–1977 Search PubMed.
- C. S. Pfeifer, Z. R. Shelton, R. R. Braga, D. Windmoller, J. C. Machado and J. W. Stansbury, Eur. Polym. J., 2011, 47, 162–170 CrossRef CAS PubMed.
- D. P. Varma, Ph.D thesis, Cochin University of Science and Technology, 2010, p. 127.
- R. Ramani and S. Alam, J. Appl. Polym. Sci., 2016, 133, 42961–42969 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07191k |
| This journal is © The Royal Society of Chemistry 2016 |