QCM-based immunosensor for determining immunoglobulin G
Yong J. Yuan*,
Qiaoying Chen and
Ji Li
Laboratory of Biosensing and MicroMechatronics, School of Materials Science and Engineering, Southwest Jiaotong University, Chengdu, Sichuan 610031, China. E-mail: yongyuan@swjtu.edu.cn; Tel: +86 28 8760 0980
First published on 13th April 2016
Abstract
A molecular dynamic (MD) simulation for investigating the effect of various mixed thiols on the efficiency of immunoglobulin G (IgG) and protein G immobilization was developed. Four different self-assembly systems were constructed from various thiol mixtures of different molar ratios of 16-mercaptohexadecanoc acid (MHDA) to 11-mercapto-1-undecanol (MUO). The simulation results were revealed that the orientation of SAM increased with the concentration of MUO. A new model for adsorption process was proposed and predicted that the surface functionalized with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixed self-assembled monolayer (SAM) exhibited the best result in terms of IgG immobilization. The surface energy of adsorption Esurface = −43.578 kcal mol−1 was obtained by simulation. In the experimental study, the results of simulation were verified by using a prototype quartz crystal microbalance (QCM) sensor. The molecules of IgG were immobilized on the functional surface with different molar ratio of thiol molecules. The shift of resonant frequency shows the biggest change Δf = 243 ± 6 Hz when the molar ratio of mixed thiol is 1
7 mixed self-assembled monolayer (SAM) exhibited the best result in terms of IgG immobilization. The surface energy of adsorption Esurface = −43.578 kcal mol−1 was obtained by simulation. In the experimental study, the results of simulation were verified by using a prototype quartz crystal microbalance (QCM) sensor. The molecules of IgG were immobilized on the functional surface with different molar ratio of thiol molecules. The shift of resonant frequency shows the biggest change Δf = 243 ± 6 Hz when the molar ratio of mixed thiol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. For further confirmation, the protein G was immobilized on the QCM surface which was functionalized with IgG. The response of resonant frequency for the system of 1
7. For further confirmation, the protein G was immobilized on the QCM surface which was functionalized with IgG. The response of resonant frequency for the system of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixed SAM also exhibited the biggest change which was 987 ± 28 Hz. These results are agreed well with the one obtained by simulation. More importantly, this study has established a new avenue of QCM chip applications.
7 mixed SAM also exhibited the biggest change which was 987 ± 28 Hz. These results are agreed well with the one obtained by simulation. More importantly, this study has established a new avenue of QCM chip applications.
1. Introduction
Organic thiols and disulfides were spontaneously chemisorbed from solutions onto noble metals such as gold and silver to form monolayer films in a very reproducible way.1–4 These films, often referred as SAM, are densely packed and possess crystalline-like structure. Due to these features, materials with new surface properties can be designed for the controlled binding of biomolecules by appropriate selection of the tail-group functionality of the alkylthiol.5 Compared with homogeneous SAM adlayers, SAM resulting from co-adsorption of two different alkanethiols has been shown to promote protein adsorption due to multiple chemical functionalities on the surfaces.6One of the most advanced applications of mixed SAMs on gold is the design of immunosensor.7 The strategy that was chosen to create the sensing layer should enable both the amount and the orientation of the baroreceptor on the transducer to be controlled and at the same time preserving its bioactivity. Numerous strategies have been designed to immobilize antibodies or antigens for immunosensor build up. Yoon et al.8 studied the immobilization of antibodies through antigen-binding site protection and immobilization kinetic control with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of two thiol molecules, i.e., 12-mercaptododecanoic acid and 1-heptanethiol. Briand et al.9 studied the effect of composition and structure of the binary thiol molecules (1
1 volume ratio of two thiol molecules, i.e., 12-mercaptododecanoic acid and 1-heptanethiol. Briand et al.9 studied the effect of composition and structure of the binary thiol molecules (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 volume ratio) on the immunosensor efficiency by using atomic force microscopy (AFM) and polarization-infrared spectroscopy (PM-IRRAS). The results demonstrated that the binding capacity of the antibody for the antigen was better for the mixed SAM of 11-mercaptoundecanoic acid (MUA) and 6-mercaptohexanol. This feature can be exploited to immobilize biomolecules in a manner which avoids steric hindrance between these molecules. Different volume ratios of mixed thiol were adopted in these literature, however, the effect of proportion on the efficiency of antibody immobilization was not considered.
3 volume ratio) on the immunosensor efficiency by using atomic force microscopy (AFM) and polarization-infrared spectroscopy (PM-IRRAS). The results demonstrated that the binding capacity of the antibody for the antigen was better for the mixed SAM of 11-mercaptoundecanoic acid (MUA) and 6-mercaptohexanol. This feature can be exploited to immobilize biomolecules in a manner which avoids steric hindrance between these molecules. Different volume ratios of mixed thiol were adopted in these literature, however, the effect of proportion on the efficiency of antibody immobilization was not considered.
The quartz crystal microbalance (QCM) is currently being investigated as a device for studying the interaction between SAM and antibodies (more specifically immunoglobulin G (IgG)).10,11 Alkanethiols of the type HS–(CH2)n–X were usually used for IgG binding. The nature of the surface formed, for example its hydrophobicity, depends largely on the tail group X, particularly when n > 10.12 Subsequent immobilization of antibodies onto the self-assembled layer can occur directly via the X moiety. Often, further chemical treatment13 is performed to activate X in order to facilitate IgG binding. To obtain an optimal surface for the immobilization of IgG, various mixed SAMs prepared with carboxylic- and hydroxyl-terminated thiol were examined in this paper. Firstly, the surface structures of various mixed thiols were simulated by using molecular dynamic program and the efficiency of antibody immobilization based on the different structures was predicted. Secondly, the theory results were verified by a homemade QCM. The next section gives the key details.
2. Simulation
2.1 Method
In this study, thiol molecules of MHDA and MUO were used as prototypical molecules in the SAM fabrication. Based on the molecular dynamic,14 the Dreiding force fields15 were adopted to model the SAM systems. To investigate the effect of various mixed SAM on the degree of antibody immobilization, the volume ratio of MHDA and MUO were set to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (SAM1), 1
1 (SAM1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (SAM2), 1
3 (SAM2), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (SAM3), and 1
5 (SAM3), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (SAM4) respectively. The total potential energy (Etotal) of thiol molecules is the sum of Einter and Eintra which represents the inter-molecular energies and intra-molecular energies, respectively. The energy of intra-molecules includes bond stretching (Ebond), bond bending (Eangle), and torsion (Edihedral) according to the follow equation:
7 (SAM4) respectively. The total potential energy (Etotal) of thiol molecules is the sum of Einter and Eintra which represents the inter-molecular energies and intra-molecular energies, respectively. The energy of intra-molecules includes bond stretching (Ebond), bond bending (Eangle), and torsion (Edihedral) according to the follow equation:| Eintra = Ebond + Eangle + Edihedral | (1) |
| Ebond = kb(r − r0)2 | (2) |
| Eangle = kθ(θ − θ0)2 | (3) |
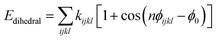 | (4) |
| Bond stretch | ||
|---|---|---|
| Bond | r0 (Å) | kb (kcal mol−1) |
| S–C | 1.81 | 450 |
| C–C | 1.54 | 200 |
| C–H | 1.08 | 300 |
| C–O | 1.42 | 600 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1.23 | 580 |
(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )C–O )C–O |
1.38 | 450 |
| OH | 0.96 | 450 |
| Angle bend | ||
|---|---|---|
| Bond | θ0 (degree) | kθ (kcal mol−1) |
| S–C–C | 112.5 | 50 |
| H–C–H | 109.5 | 40 |
| C–C–C | 111 | 45 |
C–C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
109.5 | 70 |
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
118.5 | 85 |
C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O O)–O |
120 | 85 |
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O C–O |
120 | 85 |
| C–O–H | 109.5 | 50 |
| C–C–O | 108.9 | 59 |
| Dihedral | |||
|---|---|---|---|
| Bond | ϕ0 (degree) | n | kijk (kcal mol−1) |
| S–C–C–H | 0 | 3 | 1.6 |
| S–C–C–C | 0 | 3 | 1.6 |
| H–C–C–H | 0 | 3 | 1.6 |
| C–C–C–C | 0 | 3 | 1.6 |
C–C–C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
0 | 3 | 1.6 |
C–C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
0 | 3 | 0 |
C–C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O O)–O |
0 | 3 | 0 |
| C–C–O–H | 180 | 2 | 1.8 |
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–H C–O–H |
180 | 2 | 1.8 |
Einter is the non-bonded interaction among thiol molecules, if two different atoms are separated by a distance rij. The energy is represented as Lennard Jones 12-6 potential:16
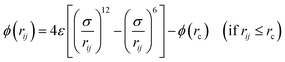 | (5) |
| Interaction | σij (Å) | kb (kcal mol−1) |
|---|---|---|
| Au–C | 3.1526 | 0.05399 |
| Au–O | 3.0145 | 0.04437 |
| Au–H | 2.7465 | 0.03142 |
| S–S | 3.5948 | 0.26200 |
| S–C | 3.4109 | 0.16762 |
| S–H | 3.1302 | 0.11780 |
| S–O | 3.3279 | 0.13842 |
| C–C | 3.4217 | 0.12510 |
| C–H | 2.9689 | 0.05899 |
| C–O | 3.2688 | 0.07816 |
| H–H | 2.4987 | 0.04125 |
| H–O | 2.7981 | 0.05023 |
| O–O | 3.09815 | 0.06000 |
The Morse potential was used to characterize the chemical bond between S and Au. The Morse potential of VAu–S is defined17 as equation:
| VAu–S(r) = De[exp(−a(r − r0)) − 1]2 − S | (6) |
| Atoms | De (kcal mol−1) | r0 (Å) | α (Å−1) | S |
|---|---|---|---|---|
| Au–S | 8.763 | 2.8 | 1.47 | 1 |
2.2 Results
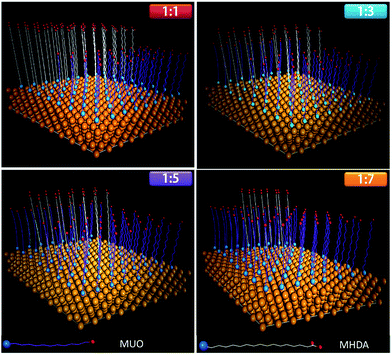
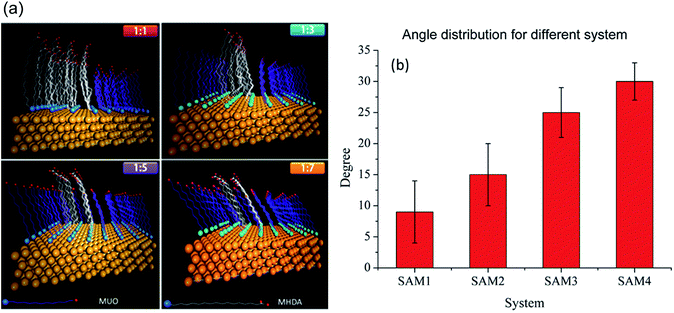
In the molecular dynamic simulation, the canonical ensemble (constant number of ligands N, volume V and temperature T) was used to model the process of thiol molecules onto gold surface. The cut-off radius was also set to 11 Å. The thermostat of Nose–Hoover18 was adopted to keep the temperature constant throughout the simulation. Atoms in the thiol molecules were assigned random initial velocities. The total time was set to 10 ns with intervals of 2 fs. Considering of phase separation and cut-off distance, the initial states of adsorption for mixed thiol were shown in Fig. 1. The thiol molecules were all perpendicularly placed on the gold surface and the distance between Au and S atoms was set to approximates 3 Å. The interval of each two sulfur atoms was set to 5 Å so that the adsorbed molecules were packed as 31/2 × 31/2 R30° on the surface.19 The area of interfacial adsorption was set as 45 Å × 45 Å, which was a periodic boundary, and the total number of thiol molecules was set as 72.After 10 ns long MD simulation, the configuration of thiol molecules underwent changes as shown in Fig. 2(a). The regularity of configuration was increased with the concentration of MUO. The distribution was more disordered when the monolayer was SAM1 and SAM2; rather, SAM3 and SAM4 have preferably orientation. The tilt angles of different monolayers were 9°, 16°, 27° and 32°, respectively, as presented in Fig. 2(b). With respect to the above mentioned tilt angles, the thicknesses of four monolayers were 23.116 Å, 22.576 Å, 21.928 Å and 21.765 Å, respectively.
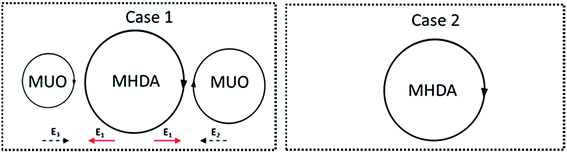
As discussed, monolayers of SAM1 and SAM2 were disordered configurations. The molecules of MHDA gathered together, leading to a large steric hindrance. According to the Yoonho et al.20 result, it is well-known that the thiol molecules often gather together spontaneously and presented as spiral. Hence, this phenomenon can be explained as two aspects as illustrated in Fig. 3:
Firstly, in the Case 1, molecules of MHDA are sandwiched by molecules of MUO. Three potential energies were hence existed. Since the directions of energies were different, the total energy of MHDA as calculated as Etotal = E1 + E2 + E3. It is therefore hard to form an order structure for MHDA. In the situation of the Case 2, there is no molecules of MUO existed around MHDA. An order structure of MHDA will be formed. With regard to an order domain, there must be a lot of molecules. This situation will therefore lead to a large steric hindrance.
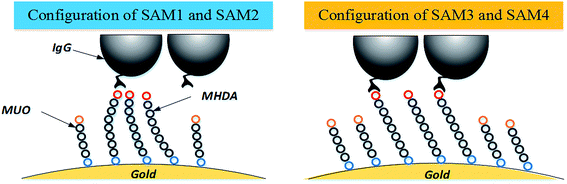
In addition to further predict the effect of various mixed SAM on the degree of antibody immobilization, a new model for adsorption process was proposed as shown in Fig. 4. After MD simulation, monolayer of SAM1 and SAM2 formed a disordered configuration. The molecules of MHDA were gathered together which led to a large steric hindrance. Hence, there were few molecules of IgG being immobilized. Conversely, SAM3 and SAM4 formed a more orderly structure. In this situation, molecules of MHDA were arranged with a certain angle and distance which provided a bigger space for MHDA to immobilize more molecules of IgG. As compared with SAM1 and SAM2, SAM3 and SAM4 were conducive to improve efficiency of immobilization for IgG.
In addition, the energy of adsorption was further investigated to obtain the most optimal parameters of concentration ratio. In the system of MD, the change of energy consisted of three parts: the total energy of system, the energy of surface and molecules. The energy of adsorption can be expressed as:
| Eadsorption = Etotal − (Esurface + Ethiol) | (7) |
The energy of adsorption reflected the degree of stability for molecules on the surface. The stability increased as the energy of adsorption decreased. Hence, the energy of adsorption for thiol molecules was calculated and the results are shown in Table 4.
| Energy (kcal mol−1) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
|---|---|---|---|---|
| Etotal | −5308.743 | −5303.318 | −5309.071 | −5313.189 |
| Esurface | −5248.417 | −5248.417 | −5248.417 | −5248.417 |
| Ethiol | −30.197 | −25.169 | −22.372 | −21.194 |
| Eadsorption | −30.129 | −34.732 | −38.282 | −43.578 |
According to the results, it is demonstrated that the energy of adsorption decreased gradually as the concentration of MUO increased. SAM1 and SAM2 have bigger adsorption energy which results in unstable surface structures. SAM4 has the lowest energy of adsorption which is −43.578 kcal mol−1. The simulated result agrees well with the one obtained by Dubois et al.21
In conclusion, the simulation results predicted that SAM4 has a great surface configuration and small steric hindrance which can be used to immobilize IgG more efficiently. In order to further verify the accuracy of simulation results, the experiments of IgG immobilization were carried out based on the homemade QCM biosensor, and the frequency responses were obtained.
3. Experimental study
3.1 Materials
All solutions were of analytical grade; ultrapure water was generated by OMNI (Research Scientific Instruments Company). Chemicals for immobilization reaction were purchased from the following sources; MUO (Sigma, USA), MHDA (Sigma, USA), 1-ethyl-3-[3-(dimethylamino) propyl]carbamide (EDC) (Sigma, USA), N-hydroxysulfosuccinimide (NHS) (Sigma, USA), and ethanolamine-HCl (Sigma, USA). Antibody used as IgG form rat serum which was obtained from Sigma.3.2 Preparation of mixed SAMs on gold surface
QCM chips were from International Crystal Manufacturing, Inc with a nominal resonance frequency of 10 MHz. The chips consisted of a circular piezoelectric crystal (about 5 mm in diameter) sandwiched between gold electrodes. According to the Sauerbrey relationship,22 an increase in the mass on the gold surface would cause the oscillation frequency to decrease. Hence, the frequency change caused by the self-assembled monolayer was utilized to determine kinetics of adsorption processes. In this study, 1 Hz of a measured frequency (in gas phase) corresponds to 1.36 ng of a mass increase.Prior to use, all bare QCM chips were exposed to a freshly prepared piranha solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, concentrated H2SO4: 30% H2O2) for 30 min, and then rinsed in ultrapure water followed by ethanol. Finally, chips were dried by high purity nitrogen. According to the previous studies, the QCM chips were modified with the mixed SAM solutions for 4 hours at 60 °C (ref. 23) and then rinsed with ethanol and ultrapure water.
1, concentrated H2SO4: 30% H2O2) for 30 min, and then rinsed in ultrapure water followed by ethanol. Finally, chips were dried by high purity nitrogen. According to the previous studies, the QCM chips were modified with the mixed SAM solutions for 4 hours at 60 °C (ref. 23) and then rinsed with ethanol and ultrapure water.
3.3 Antibody immobilization
All experiments were carried out at 25 °C unless otherwise stated. The terminal carboxylic groups of the mixed SAMs were activated for 10 min in a freshly prepared 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of EDC (0.4 M) and NHS (0.1 M) in ultrapure water. After activation, 1 mg ml−1 of rat IgG was applied and allowed to react for 30 min. Following antibody immobilization, the surface was deactivated by 0.2 M ethanolamine-HCl for 10 min to block remaining NHS-ester groups and minimize nonspecific effects. Finally, the ultrapure water was used to flush over the surface and dried with nitrogen flow.
1 mixture of EDC (0.4 M) and NHS (0.1 M) in ultrapure water. After activation, 1 mg ml−1 of rat IgG was applied and allowed to react for 30 min. Following antibody immobilization, the surface was deactivated by 0.2 M ethanolamine-HCl for 10 min to block remaining NHS-ester groups and minimize nonspecific effects. Finally, the ultrapure water was used to flush over the surface and dried with nitrogen flow.
3.4 Apparatus
A prototype bond-rupture device24 was used to carry out frequency measuring experiments. The resonant frequency of QCM chips was detected in three stages. The following 3 resonant frequencies were measured: (1) the resonant frequency f0 of the cleaned bare chips, (2) the resonant frequency f1 of chips immobilized mixed thiol, (3) the resonant frequency f2 of chips immobilized IgG, and (4) the shift resonant frequencies Δf of QCM chips were obtained by the difference between f0 and f1, f0 and f2.3.5 Results and discussion
The SAM technique represents a powerful and attractive strategy which provides well-defined and controlled structures of monomolecular interfaces of biological elements on a variety of substances. This technique was employed to functionalize the QCM sensor chip in this study. In a QCM-based biosensor, the shift of resonant frequency is related to the amount of immobilized mixed thiol molecules and IgG on the sensor surface. Therefore, to obtain an optimal surface for immobilization of the IgG antibody, four surfaces constructed by mixing different molar ratios of thiol were examined. MHDA was used to anchor the antibodies, while MUO was used to form a stable non-fouling background.Fig. 5(a) shows the effect of different molar ratio of mixed thiol on shift resonant frequency which is directly related to the IgG immobilization onto one side of the gold surface. It was revealed that the shift of resonant frequency was not affected by the different volume ratios of mixed thiol. The shift resonant frequencies were 39 ± 5 Hz, 40 ± 5 Hz, 42 ± 5 Hz and 40 ± 5 Hz when the monolayer was SAM1, SAM2, SAM3 and SAM4, respectively. According to the previous study,23 it was known that the area of gold electrodes possessed a certain value and the thiol molecules were packed with 3 × 3 R30° on the gold surface. Hence, the shift of resonant frequency of mixed thiol would not be changed in the full coverage situation. In addition, the shift of resonant frequency was about 40 Hz when the monolayer formed. This result demonstrated that the monolayer was formed on the gold surface and laid the foundation for further IgG immobilization.
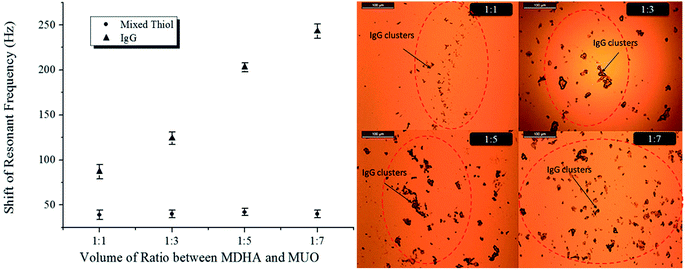 | ||
| Fig. 5 (a) The shift resonant frequency of various mixed thiol and IgG immobilization. (b) IgG distribution on the electrode of QCM. | ||
The rat IgG was a bio-macromolecule which results significant changes in the shift of resonant frequencies. The vibration of resonant frequencies increased to 87 ± 8 Hz, 124 ± 7 Hz, 203 ± 8 Hz and 243 ± 6 Hz when the molar ratio of mixed thiol were SAM1, SAM2, SAM3 and SAM4, respectively. Fig. 5(b) show the microscope photo of macro-distribution of IgG cluster on the gold surface. It was demonstrated that the amount of IgG immobilized on the gold electrode increased with addition of more MUO, thus further indicated that higher coverage of IgG was obtained. According to the Sauerbery equation, the mass on the surface is proportional to variation of frequency. Hence, IgG immobilized on SAM4 exhibited the highest molar binding ratio. On the contrary, SAM1 possessed the most severe steric hindrance and thus impeded effective IgG binding. Therefore, the lowest binding ratio was observed with SAM1.
Protein G is an immunoglobulin-binding protein expressed in group C and G streptococcal bacterial which can form specific bond with rat IgG.25 In order to further verify the simulation results, the gold surface with IgG modification was subsequently immobilized with protein G in order to determine whether or not the IgG layer could bind/detect its bioactive partner. The protein G was coated with 2.08 μm polystyrene microspheres and diluted to 200× in PBS buffer. After 2 hours of reaction, PBS buffer was cleaned with ultrapure water and dried in nitrogen environment.
Compared with the rat IgG, protein G was a bigger bio-macromolecule and the shift of resonant frequency exceeded 600 Hz as shown in Fig. 6(a). The vibration of resonant frequencies increased to 360 ± 30 Hz, 652 ± 7 Hz, 810 ± 32 Hz and 987 ± 28 Hz when the volume ratio of mixed thiol were SAM1, SAM2, SAM3 and SAM4, respectively. Fig. 6(b) show the microscope photo of distribution of protein G polystyrene microspheres on the gold surface. It was demonstrated that the amount of protein G immobilized on the gold electrode was also increased with addition of more MUO. This result is further confirmed that the efficiency of IgG immobilization increased with addition of more MUO in the mixed thiol. It was found that the SAM with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (MHDA
7 (MHDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MUO) exhibited the best sensitivity for IgG determination or protein immobilization. The experimental results also verified the prediction of simulation.
MUO) exhibited the best sensitivity for IgG determination or protein immobilization. The experimental results also verified the prediction of simulation.
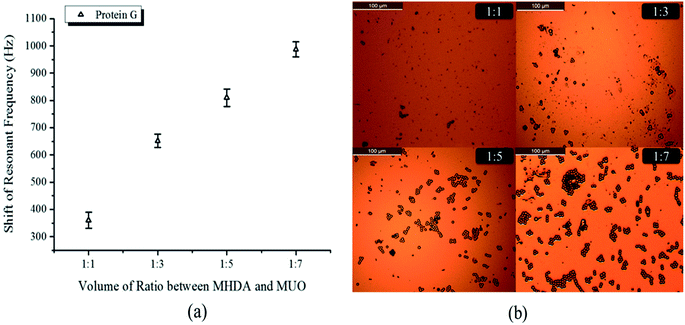 | ||
| Fig. 6 (a) The shift resonant frequency of protein G immobilization. (b) The amount of protein G polystyrene microspheres on the gold surface. | ||
4. Conclusion
In this work, the effect of various mixed SAM on the degree of antibody immobilization was studied. Simulation results obtained show that the orientation of SAM was increased with the concentration of MUO. The tilt angles of SAM1–SAM4 were 9°, 16°, 27° and 32°, respectively, and the thicknesses of monolayer were 23.116 Å, 22.576 Å, 21.928 Å and 21.765 Å, with respect to the mentioned tilt angles. The energy of adsorption was calculated to be −30.129, −34.732, −38.282 and −43.578 kcal mol−1 in the model of SAM1, SAM2, SAM3 and SAM4, respectively. A new model for adsorption process was hence proposed and predicted that SAM4 has the best configuration which can be immobilized the most amounts of IgG. In the experimental parts, the molecules of IgG were immobilized on the gold surface and obtained the shift frequencies were 87 ± 8 Hz, 124 ± 7 Hz, 203 ± 8 Hz and 243 ± 6 Hz when the molar ratio of mixed thiol was SAM1, SAM2, SAM3 and SAM4, respectively. To further confirm that the protein G was immobilized on the IgG surface and obtained the shift of resonant frequencies were 360 ± 30 Hz, 652 ± 7 Hz, 810 ± 32 Hz and 987 ± 28 Hz, respectively were obtained. Hence, the accuracy of simulation results was confirmed. In conclusion, the results of this paper will provide a theoretical platform for the study of the effect of various mixed SAM on the efficiency of antibody immobilization and also to provide a new avenue of QCM chip applications.Acknowledgements
The authors acknowledge support from National Natural Science Foundation of China under General Program Funds 30870664 and 31170954 to YJY.References
- G. N. Ralph, H. D. Lawrence and L. A. David, Fundamental studies of microscopic wetting on organic surfaces. 1. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers, J. Am. Chem. Soc., 1990, 112, 558–569 CrossRef.
- D. P. Marc, B. B. Thomas, L. A. David and E. D. C. Christopher, Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry, J. Am. Chem. Soc., 1987, 109, 3559–3568 CrossRef.
- D. B. Colin, T. Barry, T. T. Yu, E. Joseph, M. W. George and G. N. Ralph, Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold, J. Am. Chem. Soc., 1989, 111, 321–335 CrossRef.
- M. W. George and E. L. Paul, Wet chemical approaches to the characterization of organic surfaces: self-assembled monolayers, wetting, and the physical–organic chemistry of the solid–liquid interface, Langmuir, 1990, 6, 87–96 CrossRef.
- A. Ulman, Formation and Structure of Self-Assembled Monolayers, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- L. Joydeep, L. Lyle, G. Bartosz, D. C. Jeffrey and M. W. George, Biospecific Binding of Carbonic Anhydrase to Mixed SAMs Presenting Benzenesulfonamide Ligands:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Model System for Studying Lateral Steric Effects, Langmuir, 1999, 15, 7186–7198 CrossRef.
A Model System for Studying Lateral Steric Effects, Langmuir, 1999, 15, 7186–7198 CrossRef. - T. Wink, S. J. Van Zuilen, A. Bult and W. P. Van Bennekom, Self-assembled Monolayers for Biosensors, Analyst, 1997, 122, 43R–50R RSC.
- M. Yoon, H. J. Hwang and J. H. Kim, Immobilization of antibodies on the self-assembled monolayer by antigen-binding site protection and immobilization kinetic control, J. Biomed. Sci. Eng., 2011, 4, 242–247 CrossRef.
- E. Briand, M. Salmain, J. M. Herry, H. Perrot, C. Compère and C. M. Pradier, Building of an immunosensor: how can the composition and structure of the thiol attachment layer affect the immunosensor efficiency?, Biosens. Bioelectron., 2006, 22, 440–448 CrossRef CAS PubMed.
- A. Tlili, A. Abdelghani, S. Hleli and M. A. Marref, Electrical Characterization of a Thiol SAM on Gold as a First Step for the Fabrication of Immunosensors based on a Quartz Crystal Microbalance, Sensors, 2004, 4, 105–114 CrossRef CAS.
- E. Briand, M. Salmain, J. M. Herry, H. Perrot, C. Compère and C. M. Pradier, Anti-rabbit immunoglobulin G detection in complex medium by PM-RAIRS and QCM influence of the antibody immobilization method, Biosens. Bioelectron., 2007, 22, 2884–2890 CrossRef CAS PubMed.
- C. D. Bain and G. M. Whitesides, Molecular-Level Control over Surface Order in Self-Assembled Monolayer Films of Thiols on Gold, Science, 1988, 240, 62–63 CAS.
- S. Löfås and B. Johnsson, A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands, J. Chem. Soc., Chem. Commun., 1990, 21, 1526–1528 RSC.
- D. Frenkel and B. Smit. Understanding Molecular Simulation-From Algorithms to Applications, Academic Press, 1996 Search PubMed.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, DREIDING: A Generic Force Field for Molecular Simulations, J. Phys. Chem., 1990, 94, 8897–8909 CrossRef CAS.
- J. E. Lennard-Jones, On the Determination of Molecular Fields, Proc. R. Soc. London, Ser. A, 1924, 106, 463–477 CrossRef.
- P. M. Morse, Diatomic molecules according to the wave mechanics. II. Vibrational levels, Phys. Rev., 1929, 34, 57–64 CrossRef CAS.
- S. Nosé, A unified formulation of the constant temperature molecular-dynamics methods, J. Chem. Phys., 1984, 81, 511–519 CrossRef.
- C. E. D. Chidsey, G. Y. Liu, P. Rowntree and G. Scoles, Molecular Order at the Surface of an Organic Monolayer Studied by Low Energy Helium Diffraction, J. Chem. Phys., 1989, 91, 4421–4423 CrossRef CAS.
- A. Yoonho, K. S. Joyanta, C. S. George and J. Joonkyung, Molecular Dynamics Study of the Formation of a Self-Assembled Monolayer on Gold, J. Phys. Chem. C, 2011, 115, 10668–10674 Search PubMed.
- L. H. Dubois and B. R. Zegarski, Molecular Ordering of Organosulfur Compounds on Au(111) and Au(100): Adsorption From Solution and in Ultrahigh Vacuum, J. Chem. Phys., 1993, 98, 678–688 CrossRef CAS.
- G. Sauerbrey, Verwendung Von Schwingquarzen Zur Wägung dünner Schichten und zur Mikrowägung, Z. Phys., 1959, 155, 206–222 CrossRef CAS.
- J. Li and Y. J. Yuan, Physisorption and Chemisorption of a Self-Assembled Monolayer by the Quartz Crystal Microbalance, Langmuir, 2014, 9637–9642 CrossRef CAS PubMed.
- Y. J. Yuan, M. J. Van der Werff, H. G. Chen, E. R. Hirst, W. L. Xu and J. E. Bronlund, Bond Rupture of Biomolecular Interactions by Resonant Quartz Crystal, Anal. Chem., 2007, 79, 9039–9044 CrossRef CAS PubMed.
- U. Sjobring, L. Bjorck and W. Kastern, Streptococcal protein G. Gene structure and protein binding properties, J. Biol. Chem., 1991, 266, 399–405 CAS.
| This journal is © The Royal Society of Chemistry 2016 |