3D interconnected hierarchically macro-mesoporous TiO2 networks optimized by biomolecular self-assembly for high performance lithium ion batteries†
Xiao-Ning Ren‡
a,
Liang Wu‡a,
Jun Jina,
Jing Liua,
Zhi-Yi Hub,
Yu Li*a,
Tawfique Hasanc,
Xiao-Yu Yanga,
Gustaaf Van Tendeloob and
Bao-Lian Su*ade
aLaboratory of Living Materials at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 122 Luoshi Road, 430070, Wuhan, Hubei, China. E-mail: yu.li@whut.edu.cn; baoliansu@whut.edu.cn
bEMAT (Electron Microscopy for Materials Science), University of Antwerp, 171 Groenenborgerlaan, B-2020 Antwerp, Belgium
cCambridge Graphene Centre, University of Cambridge, 9 JJ Thomson Avenue, Cambridge CB3 0FA, UK
dLaboratory of Inorganic Materials Chemistry (CMI), University of Namur, 61 rue de Bruxelles, B-5000 Namur, Belgium. E-mail: bao-lian.su@unamur.be
eDepartment of Chemistry and Clare Hall, University of Cambridge, Cambridge CB21 EW, UK. E-mail: bls26@cam.ac.uk
First published on 7th March 2016
Abstract
Biomolecular self-assembly is an effective synthesis strategy for material fabrication with unique structural complexity and properties. For the first time, we integrate inner-particle mesoporosity in a three-dimensional (3D) interconnected macroporous TiO2 structure via the mediation of biomolecular self-assembly of the lipids and proteins from rape pollen coats and Pluronic P123 to optimize the structure for high performance lithium storage. Benefitting from the hierarchically 3D interconnected macro-mesoporous structure with high surface area, small nanocrystallites and good electrolyte permeation, such a unique porous structure demonstrates superior electrochemical performance, with high initial coulombic efficiency (94.4% at 1C) and a reversible discharge capacity of 161, 145, 127 and 97 mA h g−1 at 2, 5, 10 and 20C for 1000 cycles, with 79.3%, 89.9%, 90.1% and 87.4% capacity retention, respectively. Using SEM, TEM and HRTEM observations on the TiO2 materials before and after cycling, we verify that the inner-particle mesoporosity and the Li2Ti2O4 nanocrystallites formed during the cycling process in interconnected macroporous structure greatly enhance the cycle life and rate performance. Our demonstration here offers opportunities towards developing and optimizing hierarchically porous structures for energy storage applications via biomolecular self-assembly.
1 Introduction
Rechargeable lithium-ion batteries (LIBs) with a high storage capacity and cycling stability are considered to be a versatile, clean and promising energy source for next generation portable electronic devices, hybrid electric vehicles (HEV) and electric vehicles (EV).1 TiO2 is an attractive alternative to graphite due to its low volume change (3–4%)2 and relatively high lithium insertion/extraction voltage (∼1.7 V). This allows better overcharge protection and safety by avoiding the formation of solid-electrolyte interphase (SEI) layers and lithium electroplating during cycling.2 Moreover, TiO2 is suitable for energy storage owing to its abundance, low cost and low impact on environment.3Theoretically, lithium intercalating into anatase TiO2 can approach to Li0.96TiO2 composition, with a maximum capacity of ∼335 mA h g−1. However, phase transition from the original tetragonal phase to orthorhombic symmetry caused by the elastic interaction force between the intercalated lithium ions (Li+) and formation of weak Ti–Ti interactions leads to only ∼0.5 Li+ insertion per unit of TiO2 with a practical reversible capacity of 167 mA g−1.4 It has been demonstrated that the porosity in TiO2 can improve its cycling stability and capacity at high charge–discharge rates.5,6 Indeed, porous TiO2 structures, with their high surface area, narrow pore-size distribution and good electrolyte permeation have attracted significant interests for LIBs.7–11 Assembling nanosized TiO2 such as nanofibers,12 nanorods and nanoparticles12–14 to hierarchically porous micro/nanostructures is therefore a very effective approach toward the development of high performance TiO2 electrodes.15
In the context of hierarchically porous structure design, mother nature has already provided us with optimized one-, two-, and three-dimensional (1D, 2D and 3D) elaborate architectures, ranging from nanoscale to macroscale,16 inspiring us to design novel hierarchically porous materials via biotemplating.17,18 In general, pollen coats cover the surface of pollen grains and are typically macroporous structures.19 The lipids and proteins in pollen coats not only allow self-assembly of biomolecules for the formation of hierarchical biostructures, but also bind metal ion precursors for the fabrication of organic or inorganic nanostructures.20–22 It is widely known that lipids and proteins can self-assemble to porous membranes under appropriate conditions.19 It is therefore possible that the lipids and proteins in pollen coats can self-assemble to porous structures. In our previous study, we showed that the inner-particle mesoporosity in 3D ordered macro-mesoporous (3DOMM) TiO2 can improve cycle life and rate capability for LIBs by 14.3% at 1C for 200 cycles and 10.1% at a rate density of 1C, respectively.23 Therefore, it is conceivable that the optimizing hierarchically structured porous TiO2 via self-assembly of the lipids and proteins from pollen coats should offer a new material synthesis strategy for advanced LIBs.
Herein, we report synthesis of 3D interconnected macroporous TiO2 (RPC-P TiO2) with inner-mesopores via biomolecule-mediated self-assembly of the lipids and proteins from rape pollen coats (RPC) (Scheme 1A). This optimized unique, hierarchical structure with inner-particle mesopores in macroporous structure of RPC-P TiO2 demonstrates superior electrochemical performance, with high initial coulombic efficiency (94.4% at 1C) and a discharge capacity of 161, 145, 127 and 97 mA h g−1 at 2, 5, 10 and 20C after 1000 cycles, with a 79.3%, 89.9%, 90.1% and 87.4% capacity retention, respectively, for the same battery. The as-prepared RPC-P TiO2 material demonstrates much better performance than our previous work23 and other reported works for LIBs.12–14,24
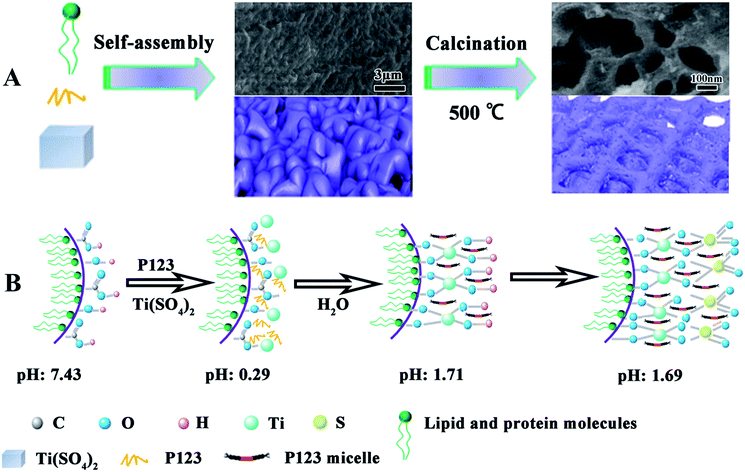 | ||
| Scheme 1 (A) Schematic illustration of the preparation process of RPC-P TiO2; (B) schematic illustration of the self-assembly process. The grey images in (A) are the corresponding SEM images. | ||
2 Experimental section
Synthesis of RPC-P TiO2
Rape pollen (5 g) is dispersed in anhydrous ethanol (50 ml) and continuously stirred for 24 h. After infiltration, the slight yellow solution containing pollen coats is obtained. The titania precursor solution is produced by dissolving Ti(SO4)2 (8 g) into anhydrous ethanol (100 ml). First, 0.4 g Pluronic P123 surfactant is dissolved into 40 ml pollen coat solution. Next, the titania precursor solution (10 ml) is poured into the 40 ml pollen coat solution, followed by continuous stirring for 2 h. Distilled water (40 ml) is then added into the solution, immediately forming reddish brown floccules. The homogeneous mixture is then transferred into Petri dishes. The dishes are kept at room temperature for 12 h and then dried at 40 °C in air for 24 h. Finally, the black brown floccules are transferred to oven at 500 °C for 6 h. After the oven is cooled down naturally at room temperature, white powders are collected for further characterization and experimentation.Synthesis of RPC TiO2
The above process followed for RPC-P TiO2 is also used to synthesize RPC TiO2. The only difference is that P123 is not added in the pollen coat solution.Characterizations
The crystalline structure of the samples is collected using powder XRD (Bruker D8 Advanced diffractometer, Cu Kα radiation, λ = 1.54056 Å). Field emission scanning electron microscopy (FESEM, Hitachi S-4800), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) are carried out on a FEI Titan 80-300 “cubed” microscope with double-correctors operated at 300 kV (For RPC-P TiO2) and JEM-2100F operated at 200 kV (For RPC-TiO2). Nitrogen adsorption–desorption isotherms are obtained using a Tristar II 3020 surface area and porosity analyzer (Micromeritics, USA). The specific surface area and pore-size distribution are calculated by the Brunauer–Emmett–Teller (BET) method and Barret–Joyner–Halenda (BJH) method, respectively.Electrochemical characterization
The working electrode is fabricated by mixing the active material (as-prepared TiO2), acetylene black, and polyvinylidene fluoride (PVDF) binder in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio. The resulting slurry is pressed and dried at 120 °C for 12 h under vacuum. CR2025 coin-type cells are assembled in a glove box under argon atmosphere. The geometrical area of the electrode is 2.0 cm2 and the weight of the active material is ∼5 mg. Lithium foil is used as both the counter and reference electrodes. A 1 M solution of LiPF6 in ethylene carbonate and dimethyl carbonate (1
1 weight ratio. The resulting slurry is pressed and dried at 120 °C for 12 h under vacuum. CR2025 coin-type cells are assembled in a glove box under argon atmosphere. The geometrical area of the electrode is 2.0 cm2 and the weight of the active material is ∼5 mg. Lithium foil is used as both the counter and reference electrodes. A 1 M solution of LiPF6 in ethylene carbonate and dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) is used as the electrolyte with a celgard membrane as the separator. The charge–discharge experiments are performed using a LAND battery tester (CT2001A) within a voltage range of 1–3 V (vs. Li/Li+) at various current densities. Electrochemical impedance spectra (EIS) are measured with an electrochemical workstation (Autolab PGSTAT 302N) from 100 kHz to 10 mHz.
1 in volume) is used as the electrolyte with a celgard membrane as the separator. The charge–discharge experiments are performed using a LAND battery tester (CT2001A) within a voltage range of 1–3 V (vs. Li/Li+) at various current densities. Electrochemical impedance spectra (EIS) are measured with an electrochemical workstation (Autolab PGSTAT 302N) from 100 kHz to 10 mHz.
3 Results and discussion
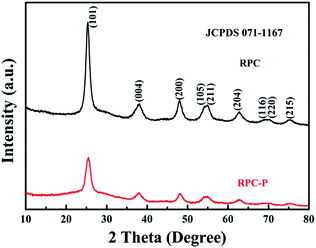
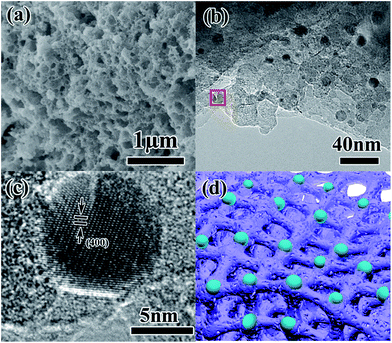
It is known that the organic groups, in particular, the hydroxyls in the pollen coat can interact with metal ions, leading to a selective growth in the framework.25 In our case, the Ti4+ first adsorbs on the surface of the pollen coat and gradually converts to titanium hydroxide as water is added in the reaction system. During the reaction process, P123 can form micelles to insert into the formed titanium hydroxides on the pollen coat, leading to inner-particle mesoporous structure (Scheme 1B). Finally, 3D optimized hierarchically macro-mesoporous TiO2 structure can be formed via self-assembly of the lipids and proteins from the rape pollen coats.Fig. 1a presents the SEM image of RPC-P TiO2, showing the 3D interconnected macroporous structure. The average dimension of the interconnected macropore is ∼100 nm. Fig. 1b displays the small nanoparticle-constructed macroporous structure. Low magnification TEM image shows that the nanoparticles tightly aggregate to form the inner-particle mesopores in the 3D interconnected macroporous structure (Fig. 1c), which is quite different from the inter-particle constructed mesoporous structure (also called worm-like mesopores).23 The nanoparticles have an average size of ∼8 nm. The HRTEM image (Fig. 1d) clearly demonstrates the mesopores inside the nanoparticles, revealing the existence of inner-particle mesoporosity. The selected area electron diffraction (SAED) pattern in Fig. 1d inset shows the (200), (103) and (101) crystal planes of anatase TiO2, consistent with the XRD results (Fig. 2).
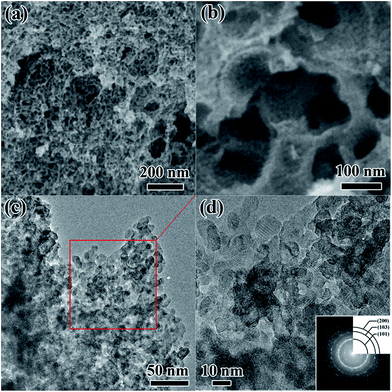 | ||
| Fig. 1 (a, b) SEM, (c) TEM and (d) HRTEM images and SAED pattern (inset) of the as-synthesized RPC-P TiO2. | ||
The N2 isotherm of RPC-P TiO2 with a type H3 hysteresis loop indicates the mesoporous structure (Fig. 3a).26 The BET specific surface area is 129.8 m2 g−1, which is higher than the TiO2 prepared with electrospinning technique method of 34–39 m2 g−1,12 and Melton salts method of 80 m2 g−1.13 The pore size distribution is ∼4 nm (Fig. 3b). Such unique inner-particle mesopores inside the macroporous structure can facilitate the electrolyte permeation, leading to a fast transportation of Li+ ions.27,28 Indeed, with the small crystallite-constructed 3D interconnected macropores and inner-particle mesopores, RPC-P TiO2 offers a bicontinuous transport path and a short path length for Li+ diffusion. The mesoporosity in RPC-P TiO2 is also capable of supporting the structure under volume variation during the Li+ insertion and extraction, resulting in a relatively high reversible capacity and excellent cycling performance.28–31
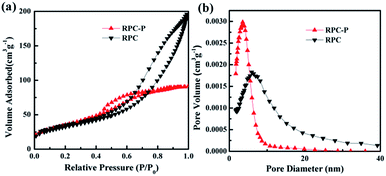 | ||
| Fig. 3 (a) N2 adsorption–desorption isotherms and (b) BJH pore size distribution plots of the as-prepared RPC TiO2 and RPC-P TiO2, respectively. | ||
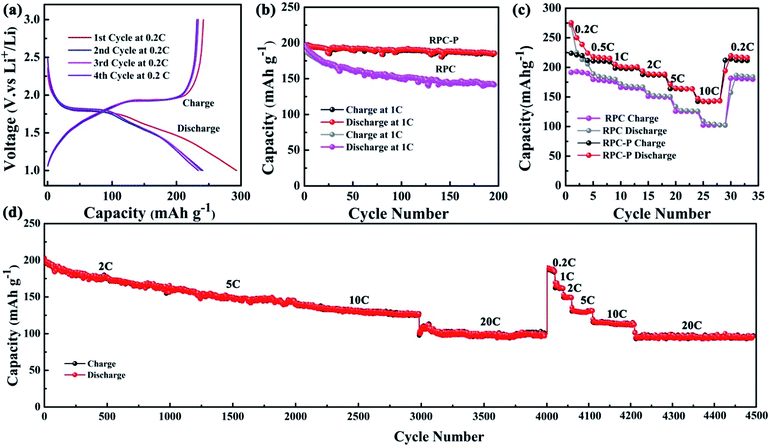
The electrochemical performance of RPC-P TiO2 is evaluated in CR2025 coin-type cells. Fig. 4a presents the charge and discharge profiles at a current density of 0.2C (1C = 167 mA g−1) for the first four cycles. The plots exhibit two obvious voltage plateaus at ∼1.75 and ∼1.95 V during the discharge and charge processes for cathodic and anodic scan, respectively, corresponding to Ti4+/3+ redox couple. The Li+ insertion process can be divided into four stages.32 The first stage corresponds to a small amount of topotactic insertion into the surface structure of the anatase, depending on its crystallite size and surface area. The second stage at ∼1.75 V represents Li+ insertion into the interstitial sites of TiO2 crystal structure. The number of Li+ ions inserted into the crystalline lattice of anatase is determined by the length of biphasic region.23 The last two stages can be divided into two regions (1.75–1.5 V and 1.5–1.0 V), showing gradual decay of the voltage after the plateau region, representing further topotactic insertion forced by the applied potential.23 The reverse process takes place during Li+ extraction. During the initial charge process, a minor difference is observed between 1.6–1.95 V for the as-prepared RPC-P TiO2 due to different Li de-insertion reaction from the amorphous nature of TiO2.13 The initial 293 mA h g−1 discharge capacity and the subsequent 242 mA h g−1 charge capacity shows 17.5% reversible capacity loss, much lower than the best values reported in literature of ∼24.2% capacity loss.33,34 The discharge and charge capacities for the second cycle are 241 and 234 mA h g−1, respectively, leading to a high coulombic efficiency of 97%. The coulombic efficiency is further increased to 98% for the third cycle and to 99.6% for the fourth cycle. Such high coulombic efficiency for the initial cycles indicates fast Li+ insertion and extraction balance in the discharge–charge processes in the macro-mesoporous RPC-P TiO2.
Fig. 4b presents the cycling performance of RPC-P TiO2 at 1C for 200 cycles. We obtain an initial discharge and charge capacity of 209 and 197 mA h g−1, respectively, resulting in an initial coulombic efficiency of 94.4%. After the second cycle, the coulombic efficiency is increased to ∼100%. Fig. S1† shows the discharge and charge profiles of RPC-P TiO2 at 1C for the 1st, 50th, 100th and 200th cycles, demonstrating very stable capacities. A reversible discharge capacity of 186 mA h g−1 is maintained after 200 charge–discharge cycles, representing a high 88.8% capacity retention. The coulombic efficiency is ∼100% during the charge–discharge process. These results indicate that the 3D interconnected macro-mesoporous TiO2 structure via biomolecular self-assembly optimization is very suitable for high rate performance LIBs.
Fig. 4c plots the rate performance of RPC-P TiO2 electrode at various charge–discharge rates. The corresponding reversible discharge capacities at 0.2, 0.5, 1, 2, 5 and 10C are 275, 218, 201, 189, 165 and 143 mA h g−1, respectively. A reversible capacity of 220 mA h g−1 is achieved when discharge capacity is moved back to 0.2C. We attribute the high capacities at various rates to the small crystallites and high specific surface area with inner-particle mesoporosity in macroporous RPC-P TiO2, leading to a shorter path length, large number of bicontinuous transport path and good contact with the electrolyte for Li+ diffusion and insertion.
To verify the advantage of the inner-particle mesoporosity in the 3D interconnected macroporous structure, we also synthesized the 3D interconnected macroporous TiO2 structure (RPC TiO2) with inter-particle mesopores. The XRD pattern indicates the anatase phase of RPC TiO2 (Fig. 2). SEM and TEM images show larger macropore and crystallite size with inter-particle mesoporosity (Fig. S2†). Its BET specific surface area is 115.4 m2 g−1 with an inter-particle mesopore of ∼7 nm (Fig. 3). Its surface area is also higher than previously reported TiO2,12,13 indicating that the biotemplating method is very suitable for high surface area porous nanostructure preparation. Fig. 4b also compares the cycling performance of RPC TiO2 against RPC-P TiO2 at 1C, showing a discharge capacity of 142 mA h g−1 with only 74.5% capacity retention for 200 cycles. The charge and discharge curves at the 1st, 50th, 100th and 200th cycle of RPC TiO2 are also presented in Fig. S1.† Both the discharge and charge capacity of RPC-P TiO2 are much higher than those of RPC TiO2. Fig. 4c shows the rate performance of RPC TiO2, demonstrating its lower performance compared to RPC-P TiO2. Indeed, all the results show that the electrochemical performance of 3D interconnected macroporous RPC-P TiO2 with inner-particle mesoporosity is superior to 3D interconnected macroporous RPC TiO2 with inter-particle mesoporosity.
To show the high electrochemical performance of RPC-P TiO2 for LIBs, the continuous cycle performance of RPC-P TiO2 at 2, 5, 10 and 20C for 1000 cycles is studied. At the current density of 2C, the initial discharge capacity is 203 mA h g−1. The discharge capacity is 161 mA h g−1 at the 1000th cycle, demonstrating 79.3% capacity retention. The coulombic efficiency is ∼100% after the second cycle (Fig. 4d). At the current density of 5C, the initial discharge capacity is 161 mA h g−1 and reversible discharge capacity is 145 mA h g−1 after 1000 cycles, showing 89.9% capacity retention and ∼100% coulombic efficiency. At the current density of 10C, the reversible discharge capacity is 127 mA h g−1 at the 1000th cycle with 90.1% capacity retention. The coulombic efficiency is ∼100%. At the current density of 20C, a reversible discharge capacity of 97 mA h g−1 is obtained after 1000 cycles, displaying 87.4% capacity retention. The coulombic efficiency is still ∼100%. After continuous 4000 cycles at different rates, the battery is reset to 0.2, 1, 2, 5, 10 and 20C to study the reversible ability of the RPC-P TiO2 electrode. When shift to 0.2, 1, 2, 5, 10 and 20C, the discharge capacity is 190, 165, 150, 132, 116 and 95 mA h g−1, demonstrating fabulous reversible performance. This result is much better than our previous work23 and other reported works.12–14,24 Thus, the RPC-P TiO2, with 3D interconnected macro-mesoporous structure presents not only high initial coulombic efficiency but also remarkable specific capacity, outstanding cycling stability and excellent rate performance for several thousand cycles.
To further reveal the electrochemical performance and provide dynamic process information of the RPC-P TiO2 and RPC TiO2 electrodes, electrochemical impedance spectroscopy (EIS) measurement is carried out. The EIS measurements of RPC-P TiO2 and RPC TiO2 are carried out after 10 galvanostatic discharge–charge cycles at 0.2C. The Nyquist plots of the RPC-P TiO2 and RPC TiO2 electrodes display one semicircle in the high and middle frequency regions (Fig. 5). The semicircles of RPC-P TiO2 and RPC TiO2 at the high and middle frequency regions include the surface film resistance (Rsf) corresponding to the formation of SEI layer and the charge transfer resistance (Rct) corresponding to the Li+ charge-transfer resistance at the interface between the electrode and electrolyte.35–37 As there is no SEI layer formed in TiO2, the surface resistance (Rsf) is thus negligible. Namely, the semicircles of RPC-P TiO2 and RPC TiO2 can be assigned to charge transfer resistance (Rct). The straight slope in the low frequency region corresponds to the solid-state diffusion of the lithium (Zw).23 Obviously, the RPC-P TiO2 electrode has lower resistance than the RPC TiO2 electrode. Therefore, the RPC-P TiO2 electrode exhibits a more rapid charge transfer and better performance than the RPC TiO2 electrode.38 We attribute this to the higher specific surface area, smaller nanocrystallites and the unique inner-particle mesopores in 3D macroporous RPC-P TiO2.
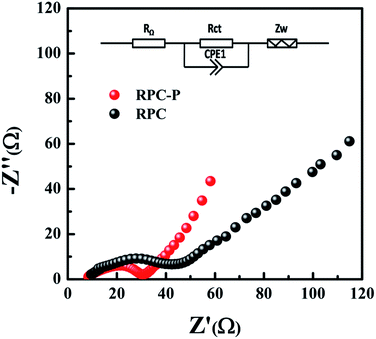 | ||
| Fig. 5 The Nyquist plots of RPC-P TiO2 and RPC TiO2 at 0.2C after 10 cycles, indicating the low Rct of the RPC-P TiO2 electrode. | ||
To understand the Li+ storage property and the stability of this structure, SEM and TEM observations of the RPC-P TiO2 electrode after 1000 cycles at 2C are performed (Fig. 6). The SEM images show that the 3D interconnected macroporous structure could still be maintained (Fig. 6a), indicating a very stable macroporous structure. Fig. S3† shows that the inner-particle mesopores are also retained in the walls of the macropores, indicating a very stable inner-particle mesoporous structure. In TEM observations, we find that after lithiation, some small nanoparticles appear in the porous structure (Fig. 6b). According to the previous report39,40 and our recent findings,41–43 the nanoparticles are Li2Ti2O4 nanocrystallites, which contribute to improvement both in capacity and rate cycling performance. Fig. 6c presents the HRTEM image of the squared Li2Ti2O4 nanoparticle shown in Fig. 6b. The well-defined lattice fringes shows a spacing of 2.09 Å, corresponding to the (400) crystal plane of cubic Li2Ti2O4 (space group: F3m3, lattice constants: a = b = c = 8.375 Å), confirming the composition of these nanocrystallites.
On the basis of the above results, we propose that the Li+ can quickly intercalate into the porous TiO2, leading to LixTiO2 formation. With further Li+ insertion into the LixTiO2 matrix, new Li2Ti2O4 nanocrystallites form in the macroporous walls as schemed in Fig. 6d. These Li2Ti2O4 crystals further facilitate the Li+ insertion capability and enhance the overall Li+ storage capacity and cycle life.41–43 The optimized unique hierarchically structured macroporous TiO2 with inner-particle mesopores and the Li2Ti2O4 nanocrystallites ensure the high reversible capacity, excellent rate capability and outstanding cycle performance.
4 Conclusions
In summary, we demonstrate a green and facile biomolecular self-assembly strategy to optimize 3D interconnected macroporous TiO2 with inner-particle mesoporosity for advanced LIBs. The RPC-P TiO2 exhibits a high initial coulombic efficiency, high capacity, long cycle life and superior electrochemical performance at high rates for several thousand cycles due to its stable, hierarchically macro-mesoporous structure and shortened path length to facilitate Li+ diffusion and insertion. The remarkable electrochemical performance indicates that this unique 3D interconnected macro-mesoporous structure via biomolecular self-assembly is a promising anode material for high performance LIBs. Our work on biomolecular self-assembly of lipids and proteins from rape pollen coats would help to understand the complex natural self-assembly systems and to extend this green, facile and economical method into biotemplated synthesis for other materials for various applications, such as gas sensing, photocatalysis and photonics.Acknowledgements
B. L. Su acknowledges the Chinese Central Government for an “Expert of the State” position in the Program of the “Thousand Talents”. Y. Li acknowledges Hubei Provincial Department of Education for the “Chutian Scholar” program. T. Hasan acknowledges funding from RAEng fellowship (Graphlex) and EPSRC IAA grant EP/K503757/1 (GRASS). G. Van Tendeloo and Z. Y. Hu acknowledge support from the EC Framework 7 program ESTEEM2 (Reference 312483). This work is realized in the frame of a program for Changjiang Scholars and Innovative Research Team (IRT_15R52) of Chinese Ministry of Education. This work is also financially supported by Hubei Provincial Natural Science Foundation (2014CFB160), International Science & Technology Cooperation Program of China (2015DFE52870) and Self-determined and Innovative Research Funds of the SKLWUT (2015-ZD-7). We also thank J. L. Xie, X. Q. Liu and T. T. Luo for TEM analysis from Research and Test Center of Materials at Wuhan University of Technology.Notes and references
- Y. Xia, Z. Xiao, X. Dou, H. Huang, X. H. Lu, R. J. Yan, Y. P. Gan, W. J. Zhu, J. P. Tu and W. K. Zhang, ACS Nano, 2013, 7, 7083–7092 CrossRef CAS PubMed.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Energy Environ. Sci., 2009, 2, 818–837 CAS.
- W. Li, Z. X. Wu, J. X. Wang, A. A. Elzatahry and D. Y. Zhao, Chem. Mater., 2013, 26, 287–298 CrossRef.
- Y. Ren, L. J. Hardwick and P. G. Bruce, Angew. Chem., Int. Ed., 2010, 122, 2624–2628 CrossRef.
- L. Kavan, J. Rathouský, M. Grätzel, V. Shklover and A. Zukal, Microporous Mesoporous Mater., 2001, 44, 653–659 CrossRef.
- P. Kubiak, T. Fröschl, N. Hüsing, U. Hörmann, U. Kaiser, R. Schiller, C. K. Weiss, K. Landfester and M. Wohlfahrt-Mehrens, Small, 2011, 7, 1690–1696 CrossRef CAS PubMed.
- H. E. Wang, H. Cheng, C. P. Liu, X. Chen, Q. L. Jiang, Z. G. Lu, Y. Y. Li, C. Chung, W. J. Zhang and J. A. Zapien, J. Power Sources, 2011, 196, 6394–6399 CrossRef CAS.
- H. E. Wang, Z. G. Lu, L. J. Xi, R. G. Ma, C. D. Wang, J. A. Zapien and I. Bello, ACS Appl. Mater. Interfaces, 2012, 4, 1608–1613 CAS.
- P. Kubiak, J. Geserick, N. Hüsing and M. Wohlfahrt-Mehrens, J. Power Sources, 2008, 175, 510–516 CrossRef CAS.
- F. Schüth, Annu. Rev. Mater. Res., 2005, 35, 209–238 CrossRef.
- D. H. Chen and R. A. Caruso, Adv. Funct. Mater., 2013, 23, 1356–1374 CrossRef CAS.
- P. Zhu, Y. Z. Wu, M. V. Reddy, A. Sreekumarn Nair, B. V. R. Chowdari and S. Ramakrishna, RSC Adv., 2012, 2, 531–537 RSC.
- M. V. Reddy, X. W. Valerie Teoh, T. B. Nguyen, Y. Y. Michelle Lim and B. V. R. Chowdari, J. Electrochem. Soc., 2012, 159, A762–A769 CrossRef CAS.
- M. V. Reddy, N. Sharma, S. Adams, R. Prasada Rao, V. K. Peterson and B. V. R. Chowdari, RSC Adv., 2015, 5, 29535–29544 RSC.
- Y. Li, Z. Y. Fu and B. L. Su, Adv. Funct. Mater., 2012, 22, 4634–4667 CrossRef CAS.
- H. Zhou, T. X. Fan and D. Zhang, ChemSusChem, 2011, 4, 1344–1387 CrossRef CAS PubMed.
- C. Sanchez, H. Arribart and M. M. G. Guille, Nat. Mater., 2005, 4, 277–288 CrossRef CAS PubMed.
- T. X. Fan, S. K. Chow and D. Zhang, Prog. Mater. Sci., 2009, 54, 542–659 CrossRef CAS.
- D. Murphy, Protoplasma, 2006, 228, 31–39 CrossRef CAS PubMed.
- F. Song, H. L. Su, J. J. Chen, W. J. Moon, W. M. Lau and D. Zhang, J. Mater. Chem., 2012, 22, 1121–1126 RSC.
- F. Song, H. L. Su, J. Han, W. M. Lau, W. J. Moon and D. Zhang, J. Phys. Chem. C, 2012, 116, 10274–10281 CAS.
- S. G. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS PubMed.
- J. Jin, S. Z. Huang, J. Liu, Y. Li, D. S. Chen, H. E. Wang, Y. Yu, L. H. Chen and B. L. Su, J. Mater. Chem. A, 2014, 2, 9699–9708 CAS.
- M. V. Reddy, S. Adams, G. T. J. Liang, I. F. Mingze, H. V. T. An and B. V. R. Chowdari, Solid State Ionics, 2014, 262, 120–123 CrossRef CAS.
- P. Cheng Wang, K. Yao, J. Zhu, X. Liu, T. Ting Lu and M. Lu, Catal. Commun., 2013, 39, 90–95 CrossRef.
- K. Sing, D. Everett, R. Haul, L. Moscou, R. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- X. N. Li, Y. C. Zhu, X. Zhang, J. W. Liang and Y. T. Qian, RSC Adv., 2013, 3, 10001–10006 RSC.
- G. L. Xu, Y. F. Xu, H. Sun, F. Fu, X. M. Zheng, L. Huang, J. T. Li, S. H. Yang and S. G. Sun, Chem. Commun., 2012, 48, 8502–8504 RSC.
- X. W. Li, S. L. Xiong, J. F. Li, X. Liang, J. Z. Wang, J. Bai and Y. T. Qian, Chem.–Eur. J., 2013, 19, 11310–11319 CrossRef CAS PubMed.
- Y. M. Sun, X. L. Hu, W. Luo and Y. H. Huang, J. Mater. Chem., 2012, 22, 19190–19195 RSC.
- W. Luo, X. L. Hu, Y. M. Sun and Y. H. Huang, ACS Appl. Mater. Interfaces, 2013, 5, 1997–2003 CAS.
- M. V. Reddy, R. Jose, T. H. Teng, B. V. R. Chowdari and S. Ramakrishna, Electrochim. Acta, 2010, 55, 3109–3117 CrossRef CAS.
- L. J. Fu, T. Zhang, Q. Cao, H. P. Zhang and Y. P. Wu, Electrochem. Commun., 2007, 9, 2140–2144 CrossRef CAS.
- H. L. Jiang, X. L. Yang, C. Chen, Y. H. Zhu and C. Z. Li, New J. Chem., 2013, 37, 1578–1583 RSC.
- M. V. Reddy, B. L. W. Wen, K. P. Loh and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 7777–7785 CAS.
- M. V. Reddy, G. Pirthvi, K. P. Loh and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2014, 6, 680–690 CAS.
- M. V. Reddy, S. Madhavi, G. V. Subba Rao and B. V. R. Chowdari, J. Power Sources, 2006, 162, 1312–1321 CrossRef CAS.
- X. L. Wu, Y. G. Guo, J. Su, J. W. Xiong, Y. L. Zhang and L. J. Wan, Adv. Energy Mater., 2013, 3, 1155–1160 CrossRef CAS.
- Q. Gao, M. Gu, A. Nie, F. Mashayek, C. M. Wang, G. M. Odegard and R. Shahbazian Yassar, Chem. Mater., 2014, 26, 1660–1669 CrossRef CAS.
- R. Cava, D. Murphy, S. Zahurak, A. Santoro and R. Roth, J. Solid State Chem., 1984, 53, 64–75 CrossRef CAS.
- J. Jin, S. Z. Huang, J. Liu, Y. Li, L. H. Chen, Y. Yu, H. E. Wang, C. P. Grey and B. L. Su, Adv. Sci., 2015, 2, 1500070 Search PubMed.
- J. Jin, S. Z. Huang, Y. Li, H. Tian, H. E. Wang, L. H. Chen, Y. Yu, T. Hasan and B. L. Su, Nanoscale, 2015, 7, 12979–12989 RSC.
- J. Jin, S. Z. Huang, J. Shu, H. E. Wang, Y. Li, Y. Yu, L. H. Chen, B. J. Wang and B. L. Su, Nano Energy, 2015, 16, 339–349 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Charge and discharge curves of RPC TiO2 and RPC-P TiO2 for 100 cycles at 1C, SEM and TEM images of RPC TiO2, HRTEM image of RPC TiO2 after 1000 cycles at 2C. Data included in this publication is available at: https://www.repository.cam.ac.uk/handle/1810/254259. See DOI: 10.1039/c6ra00332j |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |