Improvement in treatment of soak liquor by combining electro-oxidation and biodegradation†
Abstract
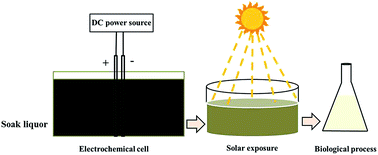
Soak liquor generated from the leather processing industry contains high organic load, making it a challenge to treat it efficiently. A combined process involving electro-oxidation and biodegradation by halophilic bacteria was applied to treat wastewater effectively for discharge. Electrolysis was performed prior to biological treatment in an electrochemical reactor at a current density of 0.012 A cm−2 for a period of 30 minutes. Titanium Substrate Insoluble Anode (TSIA) was used as an anode and titanium mesh was used as a cathode. Biological degradation was carried out with two isolated microbial strains at pH 6. The combination of electro-oxidation and biodegradation was recorded as a good technique in terms of COD (Chemical Oxygen Demand), BOD (Biological Oxygen Demand) and TKN (Total Kjeldahl Nitrogen) removal efficiency with values of 95%, 85%, and 88% respectively. The present study claims that the integrated process gives better performance for the reduction of COD when compared to previous studies.

 Please wait while we load your content...
Please wait while we load your content...