Electrochemical and surface evaluation of the anti-corrosion properties of reduced graphene oxide†
Mojtaba Bagherzadeh*a,
Zahra Shams Ghahfarokhib and
Ebrahim Ghiamati Yazdi*b
aMaterial Research School, NSTRI, 81465-1589, Isfahan, I.R. IRAN. E-mail: mjmo123@yahoo.com
bDepartment of Chemistry, Faculty of Science, University of Birjand, Birjand, I.R. IRAN. E-mail: eghiamati@birjand.ac.ir
First published on 17th February 2016
Abstract
Herein, reduced graphene oxide nanosheets (RGON) were electrochemically grown onto a carbon steel alloy from graphene oxide (GO). The produced RGON was characterized using FT-IR, Raman, XRD, SEM and TEM techniques. Then, the anti-corrosion performance of the RGON-deposited layers was evaluated by weight loss and electrochemical methods such as open circuit potential (OCP), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) in NaCl 3.5%. Covering the surface with RGON led to a negative shift in the corrosion potential (Ecorr) and a decrease in the slope of the cathodic (βc) and anodic (βa) reactions, in which both demonstrate that the RGON can hinder the corrosion reaction. Parameters affecting film formation and consequently anti-corrosion performance, such as applied voltage and deposition time, were optimized. After applying 12 V during 180 s, the formed RGON layer on carbon steel showed the lowest corrosion current density (Jcorr) and corrosion rate (CR), and the highest charge transfer resistance (Rct). Finally, the morphology of the uncovered surface and that covered by RGON after 72 h immersion in NaCl 3.5%, was studied using SEM and EDXA techniques. The observed results are presented and discussed here.
1. Introduction
Iron and iron-based alloys are extensively used in a wide variety of industrial and engineering applications, including piping and construction. Between them, carbon steels are the most famous and most popular. Due to their high mechanical strength, formability, weldability and low cost, carbon steels have been utilized in a myriad of fields such as nuclear power plants, marine utilities, chemical processing, petroleum, and construction.1 However, the major drawback of carbon steel is its limited resistance to corrosion under harsh environments. Generally, in seawater and marine solutions, corrosion of any exposed metal surfaces is inevitable and more intense.2 Certainly, presence of chloride ions in seawater induces corrosion of metals used in facility around the seacoasts that lead to economic costs.3 Therefore reducing or preventing the corrosion is very significant.4The metal surfaces can protect by painting and varnishing5 and anodizing,6 or coating with other metals or alloys,7 oxide layers,8 organic layers9 and polymers.10 Recent investigations are focused on application of novel protective nanomaterial films, with thermal and chemical stability and minimum changes in surface physical characteristics.
Graphene as a flat monolayer of carbon atoms packed into a two-dimensional (2D) honeycomb shears sliding at contact interfaces, provides corrosion protection, and reduces friction and wear.11 The high ability of graphene as a protective layer can be understood through its unique chemical and physical features.12 Recently, due to ultrathin nanostructures and chemical stability of graphene, it is applied as anticorrosive coating layers on various metal substrates to protect them from oxidation.13–21 Prasai et al. reported that formation of thin layers of graphene via mechanical transfer or electrochemical method can inhibit the metal substrate from corrosion.13 Chen et al. deposited graphene films by using chemical vapor deposition method, to improve the corrosion resistance of Cu and Cu/Ni alloys from oxidation in air.14 Sahu et al. reported a highly corrosion protective coating on Cu substrate by using graphene nanosheets.15 Raman et al. reported on ability of chemically vapor deposited graphene in protecting cooper against electrochemical degradation.16 Also, Kang et al. deposited reduced GO sheets on Fe and Cu foils to improve their oxidation resistance.17 Recently, Krishnamurthy et al. reported on employing graphene as a passivating coating material in retarding microbially-induced galvanic corrosion of metals.18 Furthermore, Huh et al. used acetone-derived graphene coating for enhancement of seawater corrosion resistance in copper.19 Mayavan et al. reported that graphene ink can play a corrosion inhibition role for iron in an aggressive chloride environment.20 And also, Singh et al. improved corrosion resistance of copper by fabrication of graphene reinforced composite coating.21
In the present work, electrochemical deposition technique was applied to deposit reduced graphene oxide nanosheets (RGON) onto a carbon steel substrate to create a protective layer. Produced RGON was characterized by using FT-IR, Raman, XRD, SEM and TEM techniques. The anti-corrosion performance of the RGON-deposited layers was evaluated by weight loss and electrochemical methods such as open circuit potential (OCP), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) in NaCl 3.5%. Observed results will be presented and discussed.
2. Experimental
2.1. Chemicals and materials
All reagents were of analytical reagent-grade and were used as supplied. Natural graphite powder, sulfuric acid, potassium peroxodisulfate, phosphorus pentoxide, phosphoric acid, potassium permanganate, hydrogen peroxide, hydrochloric acid and sodium chloride were purchased from Merck®. All solutions were prepared with purified water (18 MΩ cm, Millipore-MilliQ, Millipore Inc.). All other chemicals used were either analytical or reagent grade.2.2. Apparatuses and methods
Fourier-transform infrared (FTIR) spectroscopy analysis was performed with Nicolet 400D model. Raman spectroscopy was also performed using Almega Thermo Nicolet Dispersive Raman Spectrometer with laser: second harmonic at 532 nm of a Nd:YLF laser, and a power of 30 mW. The structure of the prepared samples was studied by X-ray diffractometer (XRD, Philips PW 1800 model). The surface morphology of the samples was observed by scanning electron microscopy, SEM equipped with EDXA, (Philips XL30 model). TEM images were obtained using transmission electron microscopy (EM 208 S 100 kW model).Weight loss measurements concerns with measuring the mass of sample before and after they exposed to aggressive medium followed by calculation the corrosion rate.22 A piece of carbon steel, its composition is summarized in Table S1 (ESI†), was cut to 0.2 × 0.5 × 1 cm3 dimensions for weight loss measurement. Prior to measurement, treatment of specimen was carried out according to Section 2.3. The specimen was weighed on a weighing balance, 0.1 mg precision, and the weight recorded. Then it is incubated in a sodium chloride corrosive solution (NaCl 3.5%). After 3 days interval (72 hours), the specimen was removed, washed with water, dried and weighed on a weighing balance to determine the weight loss during exposure. Corrosion rate in mm per year was calculated by using following equation, in ASTM practice standard G-31;23
| corrosion rate (CR) = 8.75 × 106W/ATD |
All electrochemical measurements including open circuit potential (OCP), electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization were carried out using an Autolab PGSTAT300N Potentiostat/Galvanostat equipped with a NOVA software (Eco Chemie, Utrecht, The Netherlands). A three-electrode cell including an Ag/AgCl reference electrode, a Pt electrode as the counter electrode, and a carbon steel, 0.2 × 0.5 cm (0.1 cm2), as a working electrode was used for electrochemical measurements. Anticorrosion performance was evaluated by using different electrochemical testing techniques during immersion in NaCl 3.5% corrosive solution.
For the EIS measurements, a sine wave potential with 10 mV amplitude superimposed on OCP was applied, a wide frequency range from 10 kHz to 100 MHz was scanned, and the impedances were recorded. The EIS data analysis was performed using Zview 2® software and the complex nonlinear least square (CNLS) approximation method. A modified Randles' model in which double layer capacitance (Cdl) was replaced by a constant phase element (CPE), was enough to explain the impedances obtained in the whole frequency range, from which kinetic information were extracted.24–30 The polarization curves were measured by scanning rate 10 mV s−1 in range ±200 mV around OCP. All experiments were carried out at atmospheric pressure and room temperature and performed at least thrice to ensure repeatability.
2.3. Electrode pretreatment
The working electrode with a surface area of 0.1 cm2 was prepared from carbon steel alloy. A copper wire was attached to one side of a 0.1 cm2 piece of alloy. Then, the assembly was embedded in epoxy resin, leaving the smooth side exposed. This electrode was gradually ground with different grade of emery paper, and placed in an ultrasonic bath to the water–ethanol–water for 5 minutes each, then washed with distilled water and dried with nitrogen gas. All experiments were performed using the carbon steel electrode with freshly prepared surface.2.4. Electrochemically reduction of graphene oxide
Reduced graphene oxide was electrochemically deposited on carbon steel electrodes. Graphene oxide was synthesized by the natural graphite powder according to a modified Hummers method,31 a well-known method for producing monolayered GO by liquid-phase exfoliation of graphite. An aqueous suspension of GO (1 g L−1), a carbon steel electrode as anode, a Pt wire as cathode and DC power supply model 8303, were used for the electrochemically deposition of RGON. The oxygen functional groups of GO were significantly removed by the EPD process. Possible mechanism of deoxygenation that occurred during the EPD process at the anode is well documented by Ruoff and coworkers (ESI†).32 Finally, prepared samples were removed from the solution and dried at 60 °C in an oven overnight to avoid corrosion owing to water condensation in the deposited layers.3. Results and discussion
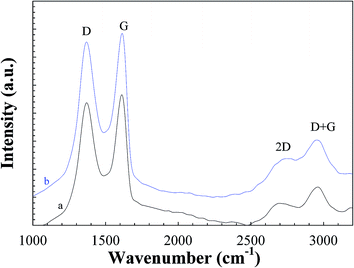
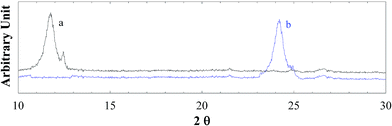
3.1. Structural and compositional analysis of GO and RGON
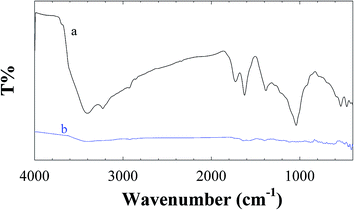
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations), 1398 cm−1 (O–H bending vibration), 1220 cm−1 (C–OH stretching vibrations), and 1042 cm−1 (C–O stretching vibrations).31 As can be seen the C
O stretching vibrations), 1398 cm−1 (O–H bending vibration), 1220 cm−1 (C–OH stretching vibrations), and 1042 cm−1 (C–O stretching vibrations).31 As can be seen the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration at 1644 cm−1 is observed for both graphene oxide and electrochemically reduced graphene oxide. However, the O–H stretching vibration at 3414 cm−1 is significantly decreased due to the deoxygenation, and characteristic absorption bands of other oxide groups are decreased obviously (Fig. 1 spectrum b). Observed result demonstrated that the GO was successfully electrochemically reduced to the RGON.
C stretching vibration at 1644 cm−1 is observed for both graphene oxide and electrochemically reduced graphene oxide. However, the O–H stretching vibration at 3414 cm−1 is significantly decreased due to the deoxygenation, and characteristic absorption bands of other oxide groups are decreased obviously (Fig. 1 spectrum b). Observed result demonstrated that the GO was successfully electrochemically reduced to the RGON.
| Sample | Composition% | |||
|---|---|---|---|---|
| Fe | C | O | C/O | |
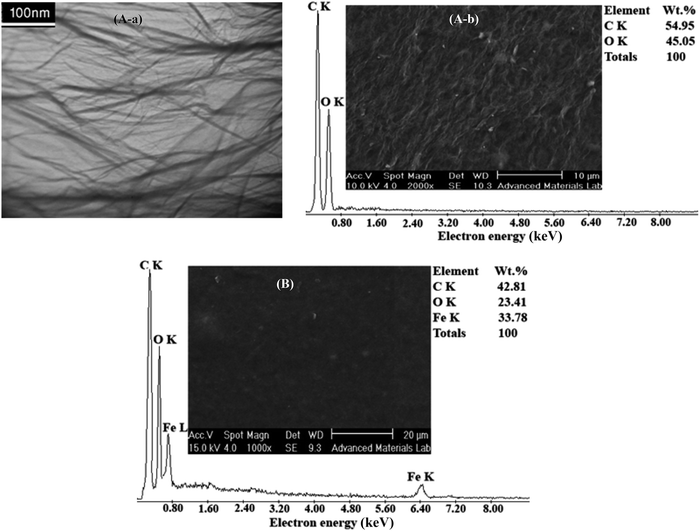
| GO | — | 54.95 | 45.05 | 1.21 |
| RGON | 33.78 | 42.81 | 23.41 | 1.82 |
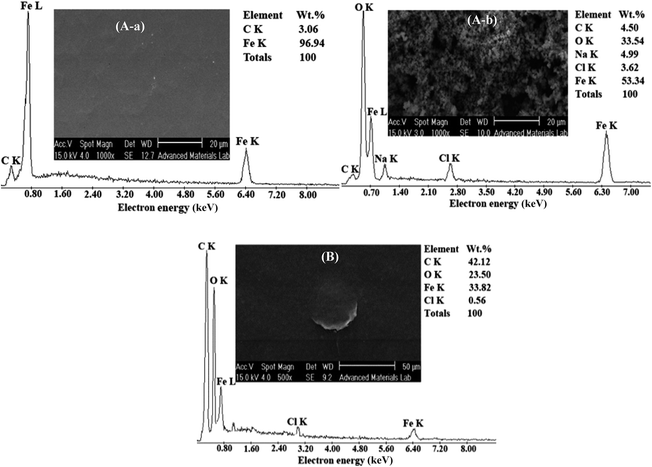
The EDX analysis and SEM image of carbon steel coated by RGON are preselected in Fig. 4B. As can be seen from SEM image, the RGONs were coated uniformly on the surface. Elemental analysis of the RGON by using EDXA shows a significant decrease in oxygen content in comparison GO, indicating GO was converted to graphene by removing the surface oxygen functional groups via electrochemical reduction. Observed results are summarized in Table 1. A little decrease in carbon content of the RGON in comparison to GO may be due to the removal of carboxylic groups during the reduction. Finally, observed increasing in C/O ratio from RGO to GO, proofs conversion of GO to RGON (Table 1).
3.2. Anticorrosion behavior of RGON
The corrosion and rust formation on steel involves several steps including oxidation/reduction reactions. However, Scheme S1† depicts the corrosion phenomenon of iron base alloy in a chloride containing environment (ESI†).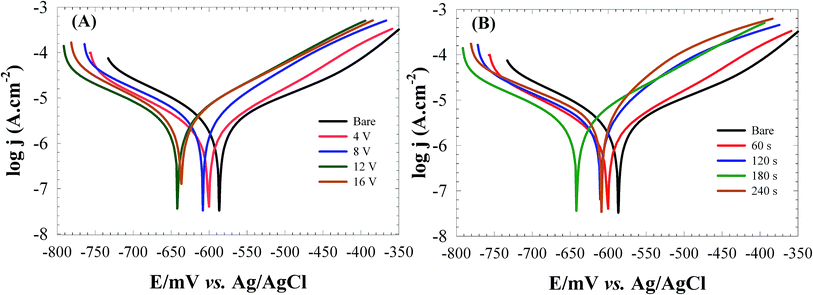 | ||
| Fig. 6 The Tafel plots for the bare and RGON-coated carbon steel at; different applied voltages during 180 s (A), and different deposition times at 12 V (B) in 3.5% NaCl solution. | ||
| Constant time (180 s) | Constant voltage (12 V) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bare | 4 V | 8 V | 12 V | 16 V | 60 s | 120 s | 180 s | 240 s | |
| Ecorr (mV) | −590 | −600 | −608 | −641 | −636 | −600 | −610 | −641 | −618 |
| Jcorr (μA cm−2) | 69.2 | 47.3 | 39.3 | 30.5 | 52.3 | 46.8 | 39.4 | 30.5 | 60.6 |
| βa (mV dec−1) | 248 | 246 | 127 | 112 | 160 | 241 | 160 | 112 | 151.4 |
| βc (mV dec−1) | 260 | 179 | 147 | 101 | 134 | 133 | 106 | 101 | 103 |
| CR (mm per year) | 0.804 | 0.550 | 0.457 | 0.354 | 0.608 | 0.544 | 0.459 | 0.354 | 0.820 |
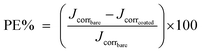
| PE% | — | 31.6 | 43.2 | 56 | 24.4 | 32.4 | 43 | 56 | 12.4 |
The protective efficiency (PE) of the coatings was determined using the following equation:
The simplest and oldest method of measuring CR in plant and equipment is weight loss analysis.22 The CR values measured from weight losing method as 0.7941 and 0.3839 mm per year were in good agreement with calculated values as 0.804 and 0.354 mm per year from Tafel plots for the bare and RGONs coated carbon steel (12 V and 180 s), respectively.
The observed decrease in corrosion rate may be due to the presence of RGONs layer on the surface which hindered the diffusion pathways for oxygen and water molecules to the carbon steel surface. As previously mentioned the process of the carbon steel corrosion involves several steps which depend on the presence of O2 and H2O. When the coating prevents O2 gas molecules from reaching the surface, the coating becomes more effective for corrosion protection.
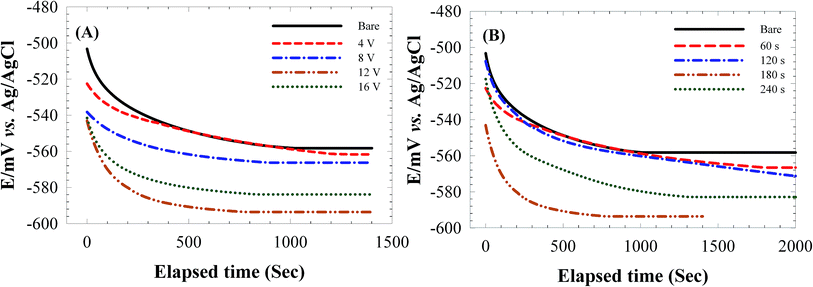
Thus, at optimized deposition conditions (12 V and 180 s), the diffusion pathway of O2 and H2O is hindered further resulting in more effective protection of carbon steel surface against corrosion. Observed results are in good agreements with OCP observations.
| Rs (Ω) | Qdl (Sn Ω−1) | n1 | Rct (Ω) | Qc (Sn Ω−1) | n2 | W (Sn Ω−1) | n3 | PE% | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Bare | 31.7(±0.3) | 7.9(±0.2) × 10−5 | 0.75 | 40.0(±0.5) | — | — | 1.7(±0.4) | 0.5 | — | |
| Applied voltages | 4 V | 31.8(±0.2) | 5.8(±0.6) × 10−5 | 0.73 | 45.6(±0.9) | 7.7(±2) × 10−4 | 0.99 | 1.3(±0.4) × 102 | 0.4 | 12.3 |
| 8 V | 27.6(±0.1) | 5.5(±0.3) × 10−5 | 0.76 | 48.2(±0.8) | 7.8(±0.2) × 10−4 | 0.66 | 1.7(±0.5) × 102 | 0.5 | 17.0 | |
| 12 V | 26.1(±0.1) | 4.2(±0.2) × 10−5 | 0.75 | 121.9(±2.5) | 8.0(±3) × 10−4 | 0.68 | 1.9(±0.3) × 102 | 0.4 | 67.2 | |
| 16 V | 28.8(±0.1) | 8.1(±0.3) × 10−5 | 0.71 | 60.8(±0.6) | 3.3(±0.1) × 10−4 | 0.67 | 7.2(±0.1) × 102 | 0.5 | 34.2 | |
| Deposition time | 60 s | 29.2(±0.1) | 2.3(±0.1) × 10−5 | 0.78 | 54.8(±0.3) | 2.4(±0.04) × 10−4 | 0.79 | 1.5(±0.6) × 102 | 0.6 | 27.0 |
| 120 s | 25.3(±0.1) | 2.8(±0.2) × 10−5 | 0.80 | 54.9(±1.0) | 4.9(±1.3) × 10−4 | 0.89 | 1.2(±0.3) × 102 | 0.4 | 27.1 | |
| 180 s | 26.1(±0.1) | 4.2(±0.2) × 10−5 | 0.75 | 121.9(±2.5) | 8.0(±3) × 10−4 | 0.68 | 1.9(±0.3) × 102 | 0.4 | 67.2 | |
| 240 s | 25.3(±0.1) | 3.2(±0.1) × 10−5 | 0.79 | 60.7(±0.3) | 7.0(±0.6) × 10−4 | 0.81 | 2.1(±0.1) × 102 | 0.5 | 34.1 |
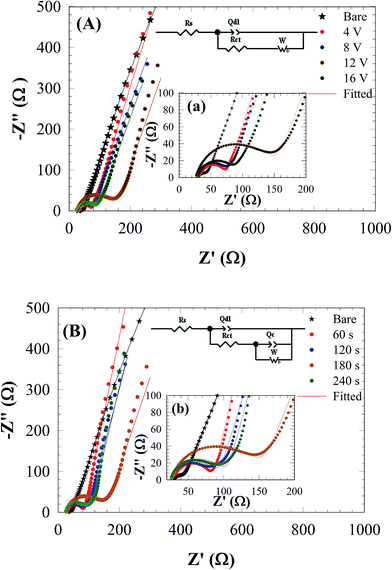
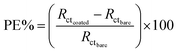
As can be seen from Fig. 7A and B in the complex plane plots of RGONs coated carbon steel samples, an increasing in the Rct and decreasing in Qdl were observed in comparison to the bare carbon steel (Table 3). The diameter of semicircle at high frequencies represents the resistance of electrochemical reactions on the electrode. It enlarges markedly for the coating samples which means provides more charge transfer resistance due to the graphene layer. The RGONs coated carbon steel at 12 V and 180 s, shows the highest Rct value as 121.9 Ω while that measured at bare carbon steel is 40 Ω. Obtained results are in good agreements with potentiodynamic polarization measurements.
The protective efficiency (PE) of samples was calculated from Rct values according to the following equation:41
3.3. Surface study of carbon steel
Fig. 8A shows the EDX analysis and SEM images (insets), of carbon steel surface before (a) and after (b) 72 h immersion in NaCl 3.5% corrosive solution. As can be seen from SEM images in Fig. 8A insets, the clean and uniform surface of carbon steel was damaged after exposure to NaCl 3.5% corrosive solution (Fig. 8A-b inset). The permeation of water, dissolved oxygen and attractive Cl− into the outer layer could result in oxidation of the whole surface. Furthermore appearing the strong O and little Cl peaks in the EDX analysis, Fig. 8A-b and Table 4, indicates that iron chloride formed on the surface and subsequently hydrolyzed to iron oxide layer (Fe2+ + 2Cl− → FeCl2, FeCl2 + H2O → FeO + 2H+ + 2Cl−).19| Sample | Composition% | |||
|---|---|---|---|---|
| Fe | C | O | Cl | |
| Bare carbon steel before corrosion | 96.94 | 3.06 | — | — |
| Bare carbon steel after corrosion | 53.34 | 4.50 | 33.54 | 3.62 |
| RGON-coated carbon steel after corrosion | 33.82 | 42.12 | 23.50 | 0.56 |
Fig. 8B inset, shows the surface of RGON coated carbon steel damaged at cracks in the RGON as corrosion initiation sites, while most of the sample surface leave undamaged. It shows RGON does not completely corrode in our experiment. The EDX results indicate existence of much lower O and Cl contents on the surface of the RGON coated sample in compared to uncoated sample (Table 4).
Decreasing in O and Cl contents, in RGON coated toward bare carbon steel after immersion in NaCl 3.5% corrosive solution, implies that products of corrosion such as FeCl2 and FeO are decreased. These data confirm the ability of RGON coating to suppression of penetrating the aggressive chlorine and dissolved oxygen ions into the underlying alloy.
4. Conclusions
In this research, the ability of RGON film as a protective layer to prevent corrosion of carbon steel has been studied. All results confirmed that the RGON layer supply protection for the carbon steel, because the coating film is well formed on the substrate and act as a barrier protector. Despite the corrosion protection role of RGON is distinguished as in the case of our present experiments, we would like to study the defects on the graphene surface which may be act as the corrosion initiation sites. So in our future studies we focus on the raising the value of corrosion resistance of carbon steel by modifying the graphene surface by aromatic molecules. This part of work is in progress.Acknowledgements
The authors gratefully acknowledge the NSTRI and University of Birjand for providing facilities for this work.References
- C. Carboni, P. Peyre, G. Beranger and C. Lemaitre, J. Mater. Sci., 2002, 37, 3715–3723 CrossRef CAS.
- L. Lv, S. Yuan, Y. Zheng, B. Liang and S. O. Pehkonen, Ind. Eng. Chem. Res., 2014, 53, 12363–12378 CrossRef CAS.
- U. Anees, S. A. Malik and I. Andijani, Desalination, 1999, 123, 205–213 CrossRef.
- M. G. Fontana, in Corrosion Engineering, Mc Graw-Hill Book Co, Singapore, 3rd edn, 1987 Search PubMed.
- D. M. Merkula, P. D. Novikov, V. N. Ivanenkov, V. V. Sapozhnikov and Y. I. Lyakhin, Oceanology, 1974, 14, 299–300 Search PubMed.
- M. Segarra, L. Miralles, J. Diaz, H. Xuriguera, J. M. Chimenos, F. Espiell and S. Pinol, Mater. Sci. Forum, 2003, 426–432, 3511–3516 CrossRef CAS.
- J. E. l. Gray and B. Luan, J. Alloys Compd., 2002, 336, 88–113 CrossRef CAS.
- V. K. Mittal, S. Bera, T. Saravanan, S. Sumathi, R. Krishnan, S. Rangarajan, S. Velmurugan and S. V. Narasimhan, Thin Solid Films, 2009, 517, 1672–1676 CrossRef CAS.
- B. V. A. Rao, M. Y. Iqbal and B. Sreedhar, Corros. Sci., 2009, 51, 1441–1452 CrossRef.
- M. I. Redondo and C. B. Breslin, Corros. Sci., 2007, 49, 1765–1776 CrossRef CAS.
- D. Berman, A. Erdemir and A. V. Sumant, Mater. Today, 2014, 17, 31–42 CrossRef CAS.
- M. Bagherzadeh and A. Farahbakhsh, Surface functionalization of Graphene, in Graphene Materials: Fundamental and Emerging Applications, Wiley, USA, 2015, pp. 25–65 Search PubMed.
- D. Prasai, J. C. Tuberquia, R. R. Harl, G. K. Jennings and K. I. Bolotin, ACS Nano, 2012, 6, 1102–1108 CrossRef CAS PubMed.
- S. Chen, L. Brown, M. Levendorf, W. Cai, S. Y. Ju, J. Edgeworth, X. Li, C. W. Magnuson, A. Velamakanni, R. D. Piner, J. Kang, J. Park and R. S. Ruoff, ACS Nano, 2011, 2, 1321–1327 CrossRef PubMed.
- S. C. Sahu, A. K. Samantara, M. Seth, S. Parwaiz, B. P. Singh, P. C. Rath and B. K. Jena, Electrochem. Commun., 2013, 32, 22–26 CrossRef.
- R. K. S. Raman, P. C. Banerjee, B. Lobo, D. E. H. Gullapalli, M. Sumandasa, A. Kumar, L. Choudhary, R. Tracz, P. M. Ajayan and M. Majumder, Carbon, 2012, 50, 4040–4045 CrossRef.
- D. Kang, J. Y. Kwon, H. Cho, J. H. Sim, H. S. Hwang, C. S. Kim, Y. J. Kim, R. S. Ruoff and H. S. Shin, ACS Nano, 2012, 9, 7763–7769 CrossRef PubMed.
- A. Krishnamurthy, V. Gadhamshetty, R. Mukherjee, Z. Chen, W. Ren, H. M. Cheng and N. Koratkar, Carbon, 2013, 56, 45–59 CrossRef CAS.
- J. H. Huh, S. H. Kim, J. H. Chu, S. Y. Kim, J. H. Kim and S. Y. Kwon, Nanoscale, 2014, 6, 4379–4386 RSC.
- S. Mayavan, T. Siva and S. Sathiyanarayanan, RSC Adv., 2013, 3, 24868–24871 RSC.
- B. P. Singh, S. Nayak, K. K. Nanda, B. K. Jena, S. Bhattacharjee and L. Besra, Carbon, 2013, 61, 47–56 CrossRef CAS.
- O. O. Daramola, B. O. Adewuyi and I. O. Oladele, J. Miner. Mater. Charact. Eng., 2011, 10, 888–903 Search PubMed.
- ASTM practice standard G-31 Standard Practice for Laboratory Immersion Corrosion Testing of Metals, ASTM International, 2004 Search PubMed.
- R. K. Shervedani and M. Bagherzadeh, Electrochim. Acta, 2008, 53, 6293–6303 CrossRef CAS.
- R. K. Shervedani, A. Farahbakhsh and M. Bagherzadeh, Anal. Chim. Acta, 2007, 587, 254–262 CrossRef CAS PubMed.
- R. K. Shervedani, S. M. Siadat-Barzoki and M. Bagherzadeh, Electroanalysis, 2010, 22, 969–977 CrossRef CAS.
- R. K. Shervedani, M. Bagherzadeh and S. A. Mozaffari, Sens. Actuators, B, 2006, 115, 614–621 CrossRef CAS.
- R. K. Shervedani and M. Bagherzadeh, Electroanalysis, 2008, 20, 550–557 CrossRef CAS.
- R. K. Shervedani and M. Bagherzadeh, Sens. Actuators, B, 2009, 139, 657–664 CrossRef CAS.
- R. K. Shervedani, M. Bagherzadeh, H. Sabzyan and R. Safari, J. Electroanal. Chem., 2009, 633, 259–263 CrossRef CAS.
- M. Bagherzadeh and M. Heydari, Analyst, 2013, 138, 6044–6051 RSC.
- S. J. An, Y. Zhu, S. H. Lee, M. D. Stoller, T. Emilsson, S. Park, A. Velamakanni, J. An and R. S. Ruoff, J. Phys. Chem. Lett., 2010, 1, 1259–1263 CrossRef CAS.
- C. Zhu, S. Guo, Y. Fang and S. Dong, ACS Nano, 2010, 4(4), 2429–2437 CrossRef CAS PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095–14107 CrossRef CAS.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401–187404 CrossRef CAS PubMed; M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. A. Cancado, A. Jorio and R. Sato, Phys. Chem. Chem. Phys., 2007, 9, 1276–1291 RSC.
- G. G. Kumar, C. J. Kirubaharan, S. Udhayakumar, C. Karthikeyan and K. S. Nahm, Ind. Eng. Chem. Res., 2014, 53(43), 16883–16893 CrossRef.
- H. Baniasadi, A. S. A. Ramazani, S. Mashayekhan and F. Ghaderinezhad, Synth. Met., 2014, 196, 199–205 CrossRef CAS.
- S. Acharya and S. N. Upadhyay, Trans. Indian Inst. Met., 2004, 57, 297–306 CAS.
- Y. Liu, J. Zhang, S. Li, Y. Wang, Z. Hana and L. Ren, RSC Adv., 2014, 4, 45389–45396 RSC.
- N. F. Atta, K. M. Amin, H. A. Abd El-Rehim and A. Galal, RSC Adv., 2015, 5, 71627–71636 RSC.
- A. B. da Silva, J. A. C. P. Gomes, E. D'Elia, M. J. C. Rezende, A. C. Pinto, B. N. M. Silva and B. V. Silva, Int. J. Electrochem. Sci., 2013, 8, 9317–9331 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26948b |
| This journal is © The Royal Society of Chemistry 2016 |