Forces between Staphylococcus aureus and human skin
Cécile
Formosa-Dague
a,
Zhuo-Han
Fu
a,
Cécile
Feuillie
a,
Sylvie
Derclaye
a,
Timothy J.
Foster
b,
Joan A.
Geoghegan
*b and
Yves F.
Dufrêne
*ac
aInstitute of Life Sciences, Université catholique de Louvain, Croix du Sud, 4-5, bte L7.07.06, B-1348 Louvain-la-Neuve, Belgium. E-mail: Yves.Dufrene@uclouvain.be
bDepartment of Microbiology, School of Genetics and Microbiology, Trinity College Dublin, Dublin 2, Ireland. E-mail: geoghegj@tcd.ie
cWalloon Excellence in Life sciences and Biotechnology (WELBIO), Belgium
First published on 6th May 2016
Abstract
Characterization of the molecular interactions between microbial cells and the human skin is essential to understand the functions of the skin microbiome, and to gain insight into the molecular basis of skin disorders. Although various molecular approaches have been used to study microbe–skin interactions, the underlying molecular forces were not accessible to study. Here we present a novel atomic force microscopy approach to localize and quantify the nanoscale interaction forces between the bacterial pathogen Staphylococcus aureus and human skin. A method combining nanoscale multiparametric imaging with single bacterial probes is developed to map simultaneously the topography and bacterial-binding properties of corneocytes at high spatiotemporal resolution. Further quantification of the forces between bacteria and corneocytes is achieved using single-cell force spectroscopy. The results show that the S. aureus–skin adhesion is strong (∼500 pN) and originates from multiple specific bonds between adhesins on the bacterial cell surface and target ligands on the corneocyte surface. Applicable to a wide variety of microbes and skin cells, our methodology offers exciting prospects for understanding the molecular details of skin colonization and infection.
Conceptual insightsThe human skin is colonized by a wide diversity of microorganisms, including bacterial, fungal and virus species. Although most skin colonizers are harmless or beneficial, some of them like Staphylococcus aureus can be implicated in skin disorders. So far, direct measurement of the molecular forces involved in microbe–skin interactions has not been possible. We present a new method combining multiparametric atomic force microscopy with single bacterial probes, that enables us to provide spatially-resolved quantitative maps of bacterial–host adhesion on skin surfaces. S. aureus exhibits strong adhesion forces towards skin corneocytes, that originate from multiple specific interactions between bacterial adhesins and target ligands on corneocytes. In future medical research, this nanoscopy tool could be used to advance our knowledge of S. aureus adhesion to corneocytes from children with eczema, where bacterial colonization exacerbates inflammation, providing molecular insight into the bacterial adhesins and skin ligands involved in the process. More broadly, the method should find broad utility for studying the molecular basis of host–microbe interactions. |
Introduction
The human skin is colonized by a wide diversity of microorganisms, including bacterial, fungal and virus species.1 Although most skin colonizers are harmless or beneficial, they can also be implicated in skin disorders. Staphylococcus aureus is a commensal Gram-positive bacterium that colonizes the skin and the nose of humans.2 This opportunistic pathogen is responsible for various disorders, including skin infections, endocarditis and septicaemia.3–5 Currently, highly virulent S. aureus strains, including methicillin-resistant S. aureus (MRSA), represent a leading cause of nosocomial infections that are difficult to eradicate. Nasal colonization is an established risk factor for S. aureus infections.6–8 Consequently, studying host–pathogen interactions during colonization of the skin, or the nasal mucosa, is important to understand the molecular basis of S. aureus-related skin infections and nasal colonisation, and may contribute to the development of new therapeutic strategies or vaccines.Attachment of S. aureus to the stratum corneum of the skin is believed to involve specific cell wall anchored (CWA) proteins (adhesins) on the bacterial cell surface, including fibronectin-binding protein A (FnBP A) and FnBPB, clumping factor A (ClfA) and ClfB, and iron regulated surface determinant A (IsdA).9,10 These adhesins bind to target ligands on corneocytes, that is, keratinized cells found on the outermost surface of the epidermis, the stratum corneum, or to proteins associated with epidermal keratinocytes. Understanding and reducing skin and nasal colonization would greatly benefit from methods capable of directly measuring bacterial–skin interactions. Here, we report the use of atomic force microscopy (AFM) to measure the nanoscale forces between S. aureus and human corneocytes. We present a new method which combines multiparametric imaging11–15 with the use of single bacterial probes, that enables us to provide, for the first time, spatially-resolved maps of bacterial–host adhesion on the surface of corneocytes, that are truly correlated with topography. As a complement, single-cell force spectroscopy (SCFS)16–18 allows us to quantify the forces between single bacteria and corneocytes. Analysis of the S. aureus Newman strain and a mutant deficient in sortase A, the enzyme responsible for anchoring CWA proteins to peptidoglycan19 (hereafter referred to as Newman and Newman srtA), indicates that S. aureus–skin adhesion involves multiple bonds between adhesins on the bacterial cell surface and specific ligands in the stratum corneum.
Results and discussion
Measuring bacterial–skin interaction forces
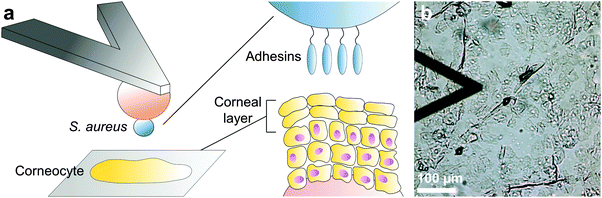
To study the forces between bacteria and human skin, force–distance curves were recorded between a bacterial cell probe and the surface of healthy human skin corneocytes immobilized on a substrate, using either multiparametric imaging or SCFS (Fig. 1). For cell probe preparation, a colloidal particle was attached to the end of a tip-less cantilever and coated with a bioinspired polydopamine wet adhesive.18 The sticky colloidal probe was used to pick up a single bacterium, and then to acquire force curves across the surface of skin corneocytes.AFM and fluorescence imaging of bacteria and corneocytes
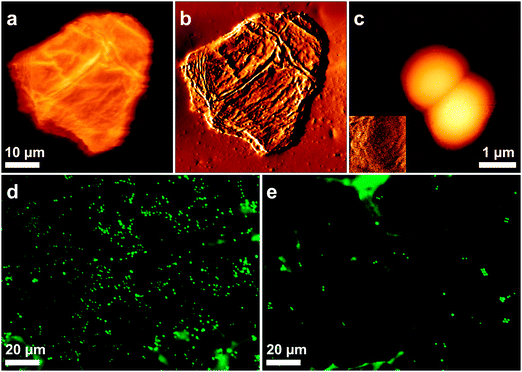
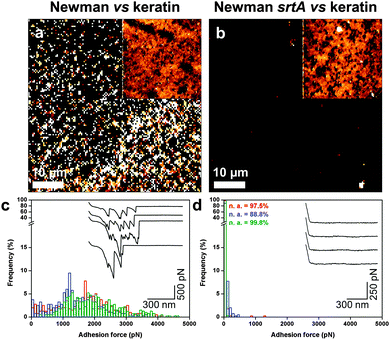
Initially, contact mode imaging with silicon nitride tips was used to visualize the surface morphology of native bacteria and skin cells in buffer solution (Fig. 2). Whereas cells from the S. aureus Newman and Newman srtA strains showed a smooth and featureless surface, corneocytes were rougher, consistent with a recent study (Fig. 2a–c).20 We also looked at bacterial–corneocyte interactions using fluorescence imaging (Fig. 2d and e). While numerous Newman bacteria were found to adhere to the corneocyte surface, this was not observed with Newman srtA cells, suggesting that CWA proteins are important for the interaction.Nanoscale imaging of the structure and adhesion of corneocytes using a single bacterial probe
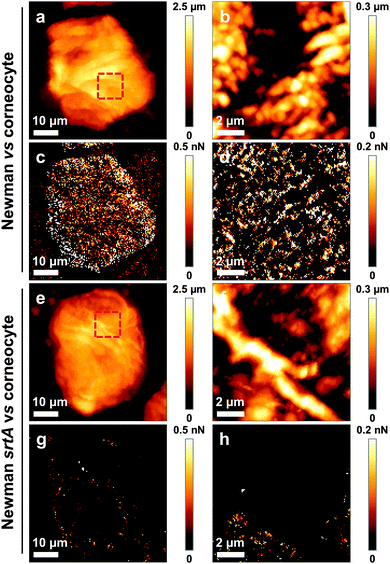
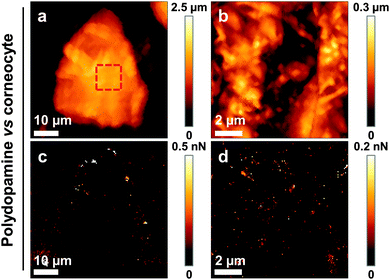
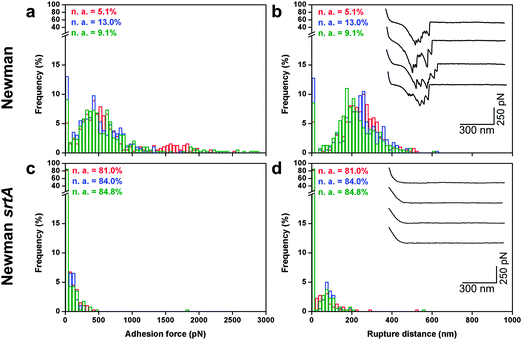
We then sought to map the location and strength of interaction between single living bacteria and the surface of corneocytes. In Fig. 3a–d, we present simultaneous AFM images of the topography and adhesion of human corneocytes recorded in PBS with a cantilever bearing a single S. aureus Newman bacterium. Strikingly, the surface structure of the corneocytes could be resolved, despite the fact that the probe was a ∼1 μm size bacterium. Adhesion images featured adhesion forces of 526 ± 21 pN (mean ± S.D. on a total of n = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 curves from 3 independent experiments) distributed across the corneocyte (bright pixels in Fig. 3c and d), while the underlying substrate showed virtually no adhesion. To confirm that polydopamine did not interfere with the measurements, and thus that only the bacterial cell was in contact with the sample, corneocytes were imaged using polydopamine-coated probes lacking bacteria. As can be seen in Fig. 4, adhesion images exhibited hardly any contrast, thus confirming that polydopamine did not contribute to the measured adhesion forces.
803 curves from 3 independent experiments) distributed across the corneocyte (bright pixels in Fig. 3c and d), while the underlying substrate showed virtually no adhesion. To confirm that polydopamine did not interfere with the measurements, and thus that only the bacterial cell was in contact with the sample, corneocytes were imaged using polydopamine-coated probes lacking bacteria. As can be seen in Fig. 4, adhesion images exhibited hardly any contrast, thus confirming that polydopamine did not contribute to the measured adhesion forces.
To gain insight into the molecular nature of the measured adhesive properties, we recorded correlated images of the structure and adhesion of corneocytes using a single S. aureus Newman srtA cell as a probe. As shown in Fig. 3e–h, most adhesion events were abolished. As the srtA mutant lacks all cell wall anchored protein adhesins, our data strongly suggest that the S. aureus–corneocyte adhesion forces are due to the specific interaction between adhesins on the bacterial cell surface and target ligands on the corneocyte surface. The method proposed here, which uniquely combines multiparametric imaging with single bacterial probes, appears to be a valuable approach to provide quantitative, spatially-resolved measurements of interaction forces between single bacteria and host surfaces, at the speed and resolution of contact mode imaging.
Strength of bacterial–skin adhesion
To provide further quantitative insights into the strength of adhesion, the interaction forces between bacteria and corneocytes were measured by SCFS. Shown in Fig. 5a and b are the maximum adhesion forces, rupture distances and representative retraction force curves obtained in PBS buffer between single S. aureus Newman bacteria and corneocytes. Most curves (90%) showed multiple adhesion events with a maximum force of 448 ± 100 pN and a rupture length of 223 ± 37 nm (n = 2000 curves from 5 different bacterium–corneocyte pairs). Adhesion was abrogated (13% adhesion on 5 different pairs) when using Newman srtA bacteria (Fig. 5c and d). Intriguingly, the binding forces measured by SCFS and multiparametric imaging were in the same range (448 vs. 526 pN), despite the fact there were differences in pulling speeds (1 vs. 40 μm s−1). This is in contrast with the notion that the binding strength of adhesion proteins, including staphylococcal adhesins, increases with the rate at which the force is applied.21–23 However, we must recall that the binding strength of such proteins also increases with the interaction time, because with time, conformational changes lead to stronger bonds and/or more proteins become engaged in adhesion.21–24 As the contact time was much longer in SCFS (100 ms vs. 0.1 ms), we speculate that pulling speed-dependent and contact time-dependent effects compensate each other, explaining the rather similar binding forces.Adhesion to keratin substrates
Finally, we investigated if the high surface roughness of corneocytes could play a role in the measured forces. High-resolution adhesion images suggest that adhesion events were not distributed homogeneously (Fig. 3d). While this heterogeneity may reflect actual variations of the corneocyte properties, it could also originate from the roughness of corneocytes, which may influence the contact area. We therefore analyzed the nanoscale forces between bacterial probes and smooth model substrates, i.e. polystyrene coated with the skin constituent keratin (Fig. 6). Adhesion images for S. aureus Newman cells featured strong adhesion forces, of 1086 ± 173 pN magnitude (3 independent samples; Fig. 6a), that were abrogated with Newman srtA bacteria (Fig. 6b). Some heterogeneity in adhesion contrast was noted, suggesting that keratin was not uniformly adsorbed on the substrate. Similarly, forces of 1467 ± 309 pN were observed by SCFS on S. aureus Newman (5 independent samples; Fig. 6c), the srtA strain showing no adhesion (Fig. 6d). These results document substantial interactions between S. aureus and smooth keratin surfaces, thus strongly supporting the validity of bacteria–corneocyte measurements.To summarize, both multiparametric and SCFS data support the notion that the adhesion between Newman bacteria and healthy corneocytes involves specific interactions between cell surface adhesins and their host ligands. We note that the ∼500 pN force is weaker than the ∼2 nN force of the SdrG–Fg bond, which involves a high-affinity dock, lock and latch (DLL) mechanism,21 thus suggesting that other binding mechanisms may contribute to the S. aureus Newman–corneocyte interaction. The force nanoscopy platform developed here could be used in future research to gain insight into the molecular basis of skin diseases such as eczema (atopic dermatitis, AD), a chronic disorder that mostly affects children.1 In particular, measuring the forces between various S. aureus strains and corneocytes from AD patients25 may help identifying the key virulence factors leading to the disease.
Conclusions
Despite the medical importance of skin–microbe interactions, measurement of the molecular forces involved has not been possible so far. Here we demonstrate that AFM techniques can readily localize and quantify adhesion forces between bacterial pathogens and their specific ligands on corneocytes. For the first time, we showed that multiparametric imaging can be performed with single bacterial probes, thus providing spatially-resolved maps of the bacterial-binding capacity of corneocytes that are truly correlated with the corneocyte surface topography, and at the same speed as that used in contact mode topographic imaging. Unlike cells from the adhesin-deficient S. aureus Newman srtA mutant, wild-type Newman cells exhibit strong adhesion forces towards corneocytes, that we believe originate from multiple specific interactions between adhesins on the bacterial cell surface and target ligands on the skin surface. Our methodology could be useful for studying the molecular basis of skin diseases such as atopic dermatitis. Measuring the forces between various S. aureus strains and corneocytes from patients may help identify the key virulence factors contributing to the disease.Methods
Bacterial strains and cultures
S. aureus strains Newman26 and Newman srtA19 were cultured in Trypticase soy broth (TSB) for 12 h at 37 °C, under agitation (180 rpm). Before experiments, cells were washed two times in pre-warmed TSB, then 100 μL of this solution was inoculated in 20 mL of fresh pre-warmed TSB. Cells were grown at 37 °C, under agitation (180 rpm), until an OD600 of 0.3 was reached.Collection of corneocytes
Corneocytes were collected by tape stripping on the volar forearm skin of a healthy volunteer. Circular adhesive tape strips (3.8 cm2, D-Squame; Monaderm, Monaco, France) were attached to the skin and pressed for 10 seconds before being removed. Eight consecutive tape strips were sampled from the same site on the skin. The first five strips were discarded and the sixth strip was placed in a closed vial and stored at 20 °C until analysis.Keratin substrates
To prepare keratin-coated surfaces, a solution of keratin 1 from human epidermis (Sigma-Aldrich) was brought in contact with a polystyrene substrate (5 μg mL−1 in PBS). Proteins were allowed to adsorb on the surface for 1 hour at room temperature, before being rinsed 10 times in PBS and further used.Fluorescence microscopy
Bacterial cells were stained using the LIVE/DEAD Baclight Viability Kit (Invitrogen, kit L7012). Cells grown to the exponential phase were washed two times in Phosphate Buffer Saline (PBS). The OD600 of the obtained cell suspension was adjusted to 1 for all experiments. According to the manufacturer instructions, 3 μL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Syto 9/IP mixture at 1.5 mM were added to 1 mL of the cell suspension, mixed thoroughly, and incubated for 15 min in the dark. The suspension was brought in contact with corneocytes and let to adhere for 30 min, after which non-adhering cells were removed by gentle rinsing with PBS. A coverslip was deposited on the corneocytes, and the system was turned upside down to allow imaging with an inverted fluorescence microscope. Optical images were recorded on a Zeiss Axio Observer Z1 equipped with a Hamamatsu camera C10600.
1 Syto 9/IP mixture at 1.5 mM were added to 1 mL of the cell suspension, mixed thoroughly, and incubated for 15 min in the dark. The suspension was brought in contact with corneocytes and let to adhere for 30 min, after which non-adhering cells were removed by gentle rinsing with PBS. A coverslip was deposited on the corneocytes, and the system was turned upside down to allow imaging with an inverted fluorescence microscope. Optical images were recorded on a Zeiss Axio Observer Z1 equipped with a Hamamatsu camera C10600.
Multiparametric imaging
Multiparametric images of corneocytes and keratin substrates were recorded in PBS using the Quantitative Imaging™ mode available on the Nanowizard III AFM (JPK Instruments, Germany). Images were obtained using a S. aureus cell probe (see below for cell probe preparation) at 128 pixels × 128 pixels, with an applied force kept at 1.0 nN, and a constant approach/retract speed of 40.0 μm s−1 (z-range of 1 μm). The cantilevers spring constants were determined by the thermal noise method.27 For each condition, experiments were repeated for at least 3 different cell pairs.Single-cell force spectroscopy
To prepare bacterial cell probes, colloidal probes were obtained by attaching a single silica microsphere (6.1 μm of diameter, Bangs Laboratories) with a thin layer of UV-curable glue (NOA 63, Norland Edmund Optics) on triangular tipless cantilevers (NP-O10, Bruker) and using a Nanowizard III AFM (JPK Instrument, Berlin, Germany). Cantilevers were then immersed for 1 h in TBS (pH = 8.5) containing 4 mg mL−1 of dopamine hydrochloride (Sigma-Aldrich), rinsed in TBS, and used directly for cell probe preparation. The nominal spring constant of the colloidal probe cantilever was of ∼0.06 N m−1, as determined by the thermal noise method.27 Then, 50 μL of a diluted cell suspension was deposited into the petri dish containing corneocytes or keratin, at a distinct location within the petri dish; 3 mL of PBS was added to the system. The colloidal probe was brought into contact with an isolated bacterium and retracted to attach the bacterial cell; proper attachment of the cell on the colloidal probe was checked using optical microscopy. Cell probes were used to measure cell–cell interaction forces at room temperature, using an applied force of 0.25 nN, a constant approach-retraction speed of 1.0 μm s−1 and a contact time of 100 ms. Data were analyzed using the Data Processing software from JPK Instruments (Berlin, Germany). Adhesion force and distance rupture histograms were obtained by calculating the maximum adhesion force and rupture distance of the last peak for each curve. For each condition, experiments were repeated for at least 5 different pairs.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
Work at the Université catholique de Louvain was supported by the National Fund for Scientific Research (FNRS), the FNRS-WELBIO under Grant no. WELBIO-CR-2015A-05, the Université catholique de Louvain (Fonds Spéciaux de Recherche), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme), and the Research Department of the Communauté française de Belgique (Concerted Research Action). Y. F. D. and C. F.-D. are respectively Research Director and postdoctoral researcher of the FNRS. We thank Orla Fleury for assistance with the preparation of tape strips and Dr Childérick Severac for help with the corneocyte imaging methodology.References
- E. A. Grice and J. A. Segre, Nat. Rev. Microbiol., 2011, 9, 244–253 CrossRef CAS PubMed
.
- A. van Belkum, N. J. Verkaik, C. P. de Vogel, H. A. Boelens, J. Verveer, J. L. Nouwen, H. A. Verbrugh and H. F. L. Wertheim, J. Infect. Dis., 2009, 199, 1820–1826 CrossRef PubMed
.
- F. D. Lowy, N. Engl. J. Med., 1998, 339, 520–532 CrossRef CAS PubMed
.
- L. McCaig, L. McDonald, S. Mandal and D. Jernigan, Emerging Infect. Dis., 2006, 12, 1715–1723 CrossRef PubMed
.
- G. J. Moran, A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey and D. A. Talan, N. Engl. J. Med., 2006, 355, 666–674 CrossRef CAS PubMed
.
- H. F. Wertheim, M. C. Vos, A. Ott, A. van Belkum, A. Voss, J. A. Kluytmans, P. H. van Keulen, C. M. Vandenbroucke-Grauls, M. H. Meester and H. A. Verbrugh, Lancet, 2004, 364, 703–705 CrossRef
.
- C. von Eiff, K. Becker, K. Machka, H. Stammer and G. Peters, N. Engl. J. Med., 2001, 344, 11–16 CrossRef CAS PubMed
.
- P. Muñoz, J. Hortal, M. Giannella, J. M. Barrio, M. Rodríguez-Créixems, M. J. Pérez, C. Rincón and E. Bouza, J. Hosp. Infect., 2008, 68, 25–31 CrossRef PubMed
.
- S. Krishna and L. S. Miller, Curr. Opin. Microbiol., 2012, 15, 28–35 CrossRef PubMed
.
- M. E. Mulcahy, J. A. Geoghegan, I. R. Monk, K. M. O'Keeffe, E. J. Walsh, T. J. Foster and R. M. McLoughlin, PLoS Pathog., 2012, 8, e1003092 CAS
.
- D. Alsteens, V. Dupres, S. Yunus, J.-P. Latgé, J. J. Heinisch and Y. F. Dufrêne, Langmuir, 2012, 28, 16738–16744 CrossRef CAS PubMed
.
- D. Alsteens, H. Trabelsi, P. Soumillion and Y. F. Dufrêne, Nat. Commun., 2013, 4, 2926 Search PubMed
.
- C. Formosa, M. Schiavone, A. Boisrame, M. L. Richard, R. E. Duval and E. Dague, Nanomedicine Nanotechnol. Biol. Med., 2015, 11, 57–65 CrossRef CAS PubMed
.
- Y. F. Dufrêne, D. Martínez-Martín, I. Medalsy, D. Alsteens and D. J. Müller, Nat. Methods, 2013, 10, 847–854 CrossRef PubMed
.
- C. Formosa-Dague, P. Speziale, T. J. Foster, J. A. Geoghegan and Y. F. Dufrêne, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 410–415 CrossRef CAS PubMed
.
- J. Helenius, C.-P. Heisenberg, H. E. Gaub and D. J. Muller, J. Cell Sci., 2008, 121, 1785–1791 CrossRef CAS PubMed
.
- A. Beaussart, S. El-Kirat-Chatel, P. Herman, D. Alsteens, J. Mahillon, P. Hols and Y. F. Dufrêne, Biophys. J., 2013, 104, 1886–1892 CrossRef CAS PubMed
.
- A. Beaussart, S. El-Kirat-Chatel, R. M. A. Sullan, D. Alsteens, P. Herman, S. Derclaye and Y. F. Dufrêne, Nat. Protoc., 2014, 9, 1049–1055 CrossRef CAS PubMed
.
- S. K. Mazmanian, G. Liu, E. R. Jensen, E. Lenoy and O. Schneewind, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 5510–5515 CrossRef CAS PubMed
.
- J. Fredonnet, G. Gasc, G. Serre, C. Séverac and M. Simon, J. Dermatol. Sci., 2014, 75, 63–65 CrossRef PubMed
.
- P. Herman, S. El-Kirat-Chatel, A. Beaussart, J. A. Geoghegan, T. J. Foster and Y. F. Dufrêne, Mol. Microbiol., 2014, 93, 356–368 CrossRef CAS PubMed
.
- P. Herman-Bausier, S. El-Kirat-Chatel, T. J. Foster, J. A. Geoghegan and Y. F. Dufrêne, mBio, 2015, 6, e00413 CrossRef CAS PubMed
.
- P. Herman-Bausier and Y. F. Dufrêne, Mol. Microbiol., 2016, 99, 611–621 CrossRef CAS PubMed
.
- C.-P. Xu, N. P. Boks, J. de Vries, H. J. Kaper, W. Norde, H. J. Busscher and H. C. van der Mei, Appl. Environ. Microbiol., 2008, 74, 7522–7528 CrossRef CAS PubMed
.
- C. Riethmuller, M. A. McAleer, S. A. Koppes, R. Abdayem, J. Franz, M. Haftek, L. E. Campbell, S. F. MacCallum, W. H. I. McLean, A. D. Irvine and S. Kezic, J. Allergy Clin. Immunol., 2015, 136, 1573–1580 CrossRef CAS PubMed
.
- E. S. Duthie and L. L. Lorenz, J. Gen. Microbiol., 1952, 6, 95–107 CAS
.
- J. L. Hutter and J. Bechhoefer, Rev. Sci. Instrum., 1993, 64, 1868–1873 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |