Precise oxygen scission of lignin derived aryl ethers to quantitatively produce aromatic hydrocarbons in water†
Abstract
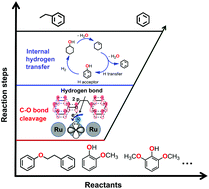
To produce aromatic hydrocarbons from biomass, a novel route is reported for one-pot selective hydrodeoxygenation of a lignin derived aryl ether mixture to C6–C9 aromatic hydrocarbons over Ru/sulfate zirconia in the aqueous phase. The cascade steps undergo the initial precise cleavage of the Caliphatic–O bond of phenolic dimers or Caromatic–OCH3 of phenolic monomers, as well as the full blockage of benzene-ring and cyclic-alkene hydrogenation to realize nearly quantitative aromatic hydrocarbon formation at 240 °C in the presence of low pressurized H2 (2–8 bar). With a Ru catalyst, the primary and competitive steps in hydrogenolysis of the C–O bond and hydrogenation of the benzene ring are shown to be sensitive to temperature and hydrogen pressure, which can subtly modify the concentration and spatial distribution of the surface adsorbed H˙. A high temperature and a low hydrogen pressure are found to be essential for hydrogenolysis, since the H˙ species nearby the oxygen atom are more strongly adsorbed under such conditions and thus preferred to be retained on the Ru surface. Herein it is defined as the “atom-induced H sorption effect”, probably resulting from the analogous hydrogen-bond force between the adsorbed H˙ and the adjacent oxygen atom on the metal surface. Besides the initial step, another key point for aromatic hydrocarbon formation is to optimize the target route of cycloalkene dehydrogenation, but suppress the parallel hydrogenation pathway to saturated cycloalkanes. It is found that the produced phenol intermediate serves as a suitable H-acceptor for the selected catalytic system during the whole conversion, via fast consumption of the in situ produced H˙ from cyclohexene dehydrogenation. Such an interesting phenomenon of internal hydrogen transfer not only promotes the dehydrogenation and hydrogenation equilibrium of cyclohexene but also lowers the in situ H˙ concentration on the Ru surface, both of which would be beneficial for reaching a high benzene yield. This hypothesis for internal hydrogen transfer is further confirmed by separate experiments and density functional theory modelling results.

 Please wait while we load your content...
Please wait while we load your content...