Facile kinetic induction of a dihydropyridide to pyrrolide ring contraction†
Abstract
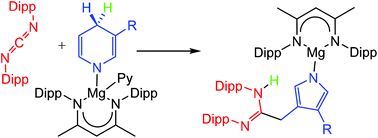
Reactions between magnesium 1,4-dihydropyridide or 1,2-dihydro-iso-quinolide derivatives and carbodiimides, RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR, generally result in Mg–N insertion and formation of guanidinate complexes. More sterically perturbed systems with N-aryl carbodiimide substitution, however, follow a divergent course of reaction initiating heterocyclic ring contraction and pyrrolide formation under unprecedentedly mild conditions.
NR, generally result in Mg–N insertion and formation of guanidinate complexes. More sterically perturbed systems with N-aryl carbodiimide substitution, however, follow a divergent course of reaction initiating heterocyclic ring contraction and pyrrolide formation under unprecedentedly mild conditions.
- This article is part of the themed collections: Main Group Transformations and Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...