Tunable reactivity of geminal bis(silyl) enol derivatives leading to selective exo-IEDDA or Sakurai allylation with a β,γ-unsaturated ketoester†
Abstract
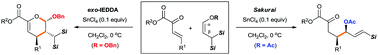
Two contrasting pathways in a SnCl4-catalyzed reaction of geminal bis(silyl) enol derivatives with β,γ-unsaturated ketoesters have been achieved by tuning the R group in the enol moiety. While the electron-donating Bn-substituted enol ether undergoes an exo-selective inverse-electron-demand Diels–Alder (IEDDA) reaction to give dihydropyran, the electron-withdrawing Ac-substituted enol ester reacts as an allylsilane to provide a Sakurai-allylated product with predominant syn-selectivity.

 Please wait while we load your content...
Please wait while we load your content...