Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Incorporation and visualization of azido-functionalized N-oleoyl serinol in Jurkat cells, mouse brain astrocytes, 3T3 fibroblasts and human brain microvascular endothelial cells†
T.
Walter‡
 a,
L.
Collenburg‡
b,
L.
Japtok
c,
B.
Kleuser
c,
S.
Schneider-Schaulies
b,
N.
Müller
b,
J.
Becam
d,
A.
Schubert-Unkmeir
d,
J. N.
Kong
e,
E.
Bieberich
e and
J.
Seibel
*a
a,
L.
Collenburg‡
b,
L.
Japtok
c,
B.
Kleuser
c,
S.
Schneider-Schaulies
b,
N.
Müller
b,
J.
Becam
d,
A.
Schubert-Unkmeir
d,
J. N.
Kong
e,
E.
Bieberich
e and
J.
Seibel
*a
aInstitute for Organic Chemistry, Julius-Maximilians University Würzburg, Am Hubland C1, Würzburg, Germany. E-mail: seibel@chemie.uni-wuerzburg.de
bInstitute of Virology and Immunobiology, Versbacher Str. 7, Würzburg, Germany
cInstitute of Nutritional Science, University of Potsdam, Arthur-Scheunert Allee 114-116, Nuthetal, Potsdam, Germany
dInstitute of Hygiene and Microbiology, Josef-Schneider-Straße 2/E1, Würzburg, Germany
eDepartment of Neuroscience and Regenerative Medicine, Medical College of Georgia, Augusta University, 1120 15th Street, Augusta, GA 30912, USA
First published on 16th June 2016
Abstract
The synthesis and biological evaluation of azido-N-oleoyl serinol is reported. It mimicks biofunctional lipid ceramides and has shown to be capable of click reactions for cell membrane imaging in Jurkat and human brain microvascular endothelial cells.
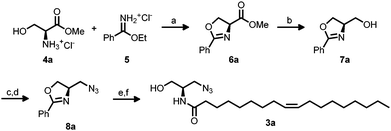
Ceramide 1 regulates physiological functions including apoptosis, cell-growth, -differentiation, -migration and adhesion and has been implicated1 in cancer, neurodegeneration, diabetes, microbial pathogenesis, obesity, and inflammation.2,3 Bieberich et al. described N-oleoyl serinol 2 (S18) as a ceramide mimicking molecule (see Fig. 1).4,5 The validity of this suggestion is reflected in the fact that it induces apoptosis in rapidly dividing neuroblastoma cells, but not in resting or differentiated cells.4 Also, 2 activates atypical protein kinase C (aPKC)4 and induces formation of aPKC associated complexes with polarity proteins in stem cell experiments.6 The similarity of S18 to ceramide is also represented by the fact that a highly specific antibody against ceramide, also developed by Bieberich et al., binds to S18.7 aPKC has been also evaluated successfully as a target for small molecule drug candidates against pancreatic cancer cells.8 Here we present preliminary data on the synthesis and biological studies of 3a and 3b azido analogues of S18. Ceramide analogs with fluorescent tags proved to be useful for the study of dynamics and visualizing membrane architecture. However, the suitability of such lipids is limited to its ability to behave similar to its unmodified counterpart. Bioorthogonal chemical reporter strategies have addressed this issue and successfully applied, i.e. the alkyne–azide “click” chemistry. In this context, we aimed to apply click chemistry to N-oleoyl serinol mimicking ceramide by introducing an azide group and examining if substitution of one serinol-hydroxyl group by an azide will preserve its biological capabilities. For the synthesis of 3 we used a modified protocol of Hawkins et al.9,10 with an enantiomer excess (ee) of 92 for 3a and 95 for 3b. For detailed Information of the synthesis see Scheme 1 and ESI.†
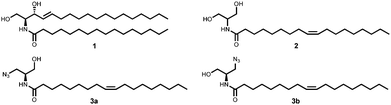 | ||
| Fig. 1 Structure of C16-ceramide 1, N-oleoyl serinol 2 and its azide-modified analogs 3a, 3b used in this work. | ||
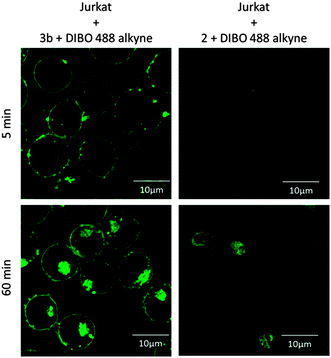
Recently the Golgi apparatus has been imaged using a clicked ceramide analogue by 3D confocal and STED microscopy in HeLa cells.11 Building on those studies, we first examined if the azido-analogues 3a and 3b of N-oleoyl serinol 2 (S18) shows cell toxicity in Jurkat T cells. Compound 3b proved to be almost non toxic at a concentration of 25 μM (Fig. S1 and S2, ESI†), comparable to the Golgi marker NBD-C6-ceramide.12 We next aimed to establish if 3b would be incorporated into the target T cells. DIBO488 dye was coupled under copper-free conditions to 3b 5 min following feeding of Jurkat cells and its accumulation at the plasma membrane was analyzed over time by flow cytometry.
While NBD-C6-ceramide included as control only slightly peaked after 25 min, this was more pronounced for 3b which continued to accumulate at the cell surface (see Fig. 2). As revealed by mass spectrometry analyses, 70% (pmol) of 3b were incorporated into Jurkat cells over time. In contrast to that seen in Jurkat cells, incorporation of 3b into unstimulated primary T cells was very low. The differential uptake was also observed upon supplementation of the labeling reaction by F-pluronic, which generally increased uptake of the azide-lipids.
To analyze whether its incorporation pattern would be compatible with that of ceramide, subcellular distribution of 3b was monitored over time in living cells. 3b efficiently accumulated at the plasma membrane and in the Golgi-compartment. Thus 3b behaves similar to the Golgi marker NBD-C6-ceramide indicating that it might truly represent a ceramide. Incorporation of the click-labeled compounds appeared to be specific as labeling intensities were dose-dependent and unconjugated, but not conjugated dye diffused into the cytosol. In addition to staining the plasma membrane, clicked 3b accumulated in an intracellular compartment also targeted by the Golgi-marker NBD-C6-ceramide (see Fig. S6 and S7, ESI†). Although both ceramide 1 and azido N-oleoyl serinol 3 insert into membranes in the similar way, they can have very different biological functions.
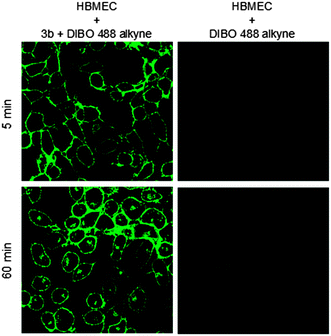
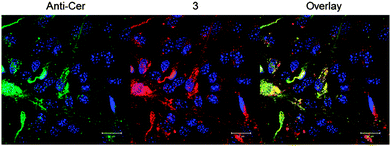
In addition to colocalization studies with NBD-C6 ceramide, we also tested colocalization of 3b with intracellular structures labeled with an antibody raised against ceramide. We used primary cultured astrocytes because they were previously shown to enrich ceramide in Golgi and other vesicular compartments.13Fig. 4 shows that there the anti-ceramide antibody labeled compartments that were also positive for 3b. Consistent with previous results obtained with anti-ceramide antibody and NBD-C6 ceramide labeling of the Golgi, these compartments partially colabeled with an antibody against GM130, a marker for cis-Golgi (see Fig. S7, ESI†). In a third approach we used human brain microvascular endothelial cells (HBMEC) as serinol acceptors. HBMEC are unique EC representatives as they have continuous intercellular tight junctions (TJs), which control movement of molecules through the EC layer. The EC–pericyte interactions lead to induction of TJ formation. Under inflammatory conditions, pericytes stimulate immune response by production of cytokines.14 Also it has been shown that Neisseria meningitidis, which targets brain ECs, induces the formation of ceramide-enriched platforms on HBMEC to favor bacterial uptake.15,16
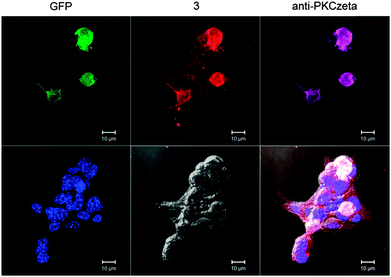
One of the fundamental biological functions of ceramides is that they can induce apoptotic cell death.17,18 HBMEC were incubated with unmodified N-oleoyl serinol 2 and the enantiomeric enriched compounds 3a and 3b and cell death were determined. Annexin V binding and propidium iodide (PI) staining enabled to discriminate between early apoptotic, late apoptotic and necrotic cell death. Treatment of HBMEC with 25 μM for 1 h did not result in any toxic effects (Fig. S2A, ESI†), however, incubation overnight (16 h) with 10 μM or 50 μM resulted in an increase of late apoptotic and in particular necrotic cells (Fig. S2B, ESI†). 3a and 3b were comparable with unmodified N-oleoyl serinol 2. They are not toxic below concentrations of 50 μM (see Fig. S2, ESI†). Bieberich et al. observed that N-oleoyl serinol 2 induces apoptosis only in rapidly dividing neuroblastoma cells. Here 3 also induced apoptosis at similar concentrations. 3b incorporation in HBMEC was efficiently (see Fig. 3). While 3b is found in the membrane after 5 min exclusively, after 60 min it is also transported in the Golgi apparatus (see Fig. 2, lower panels). We hypothesized that 3 may thus be useful in evaluation of PKCζ-mediated signal cascades. To test this hypothesis we analyzed potential interaction of the ceramide analog with PKCζ. We expressed the fusion protein human PKCζ-GFP in 3T3 fibroblasts and performed incubation and click reaction with a mixture of 3a and b. Fig. 5 shows that GFP fluorescence as well as anti-PKCζ antibody colocalized with 3a/b, suggesting that the ceramide analog interacts with PKCζ. These results indicate that 3 is useful to study the function of ceramide and its interaction with target proteins such as PKCζ for neural cell development and cell polarity. On this basis further anti-cancer strategies include the inhibition of the protein kinase C signaling pathways. For the understanding of the bacterial uptake process and the role of ceramide-enriched platforms on HBMEC these ceramide analogues might be a good tool for studying Par6 and aPKCs interactions. The Par6–aPKC interaction is necessary for the establishment and maintenance of the cell polarity complex in non-transformed cells.19 Moreover it has been shown that infection of brain ECs with N. meningitidis result in recruitment of the polarity complex Par3/Par6/Pkcζ underneath the attached bacterial microcolonies, which is followed by the delocalization of junctional proteins from the intercellular junctions.20
In summary we have demonstrated the incorporation and visualization of azido-functionalized N-oleoyl serinol by bioorthogonal click chemistry in Jurkat cells and human brain microvascular endothelial cells. We have also shown that apoptosis of the HBMEC can be titrated. It should be noted that more than half of the mitotic glia cells and neurons die during fetal brain development by apoptosis. The stained ceramide analogue can be localized. Thus it might be a helpful tool to investigate sphingosine-1-phosphate and ceramide, and their metabolites roles in endothelial barrier function, which may be a further advancement in this field.
This study was funded through the Deutsche Forschungsgemeinschaft (RU2123) and NIH grants R01 R01AG034389 and R56NS095215 (to E. B.)
Notes and references
- Y. A. Hannun and L. M. Obeid, Nat. Rev. Mol. Cell Biol., 2008, 9, 139–150 CrossRef CAS PubMed.
- Y. H. Zeidan and Y. A. Hannun, Trends Mol. Med., 2007, 13, 327–336 CrossRef CAS PubMed.
- D. Wu, Z. Ren, M. Pae, W. Guo, X. Cui, A. H. Merrill and S. N. Meydani, J. Immunol., 2007, 179, 4829–4839 CrossRef CAS.
- E. Bieberich, T. Kawaguchi and R. K. Yu, J. Biol. Chem., 2000, 275, 177–181 CrossRef CAS PubMed.
- E. Bieberich, B. Hu, J. Silva, S. MacKinnon, R. K. Yu, H. Fillmore, W. C. Broaddus and R. M. Ottenbrite, Cancer Lett., 2002, 181, 55–64 CrossRef CAS PubMed.
- G. Wang, J. Silva, K. Krishnamurthy, E. Tran, B. G. Condie and E. Bieberich, J. Biol. Chem., 2005, 280, 26415–26424 CrossRef CAS PubMed.
- K. Krishnamurthy, S. Dasgupta and E. Bieberich, J. Lipid Res., 2007, 48, 968–975 CrossRef CAS PubMed.
- A. M. Butler, M. L. Scotti Buzhardt, E. Erdogan, S. Li, K. S. Inman, A. P. Fields and N. R. Murray, OncoTargets Ther., 2015, 6, 15297–15310 Search PubMed.
- L. D. Hawkins and S. T. Ishizaka, US Pat., 20030153532, 2004 Search PubMed.
- L. D. Hawkins and S. T. Ishizaka, WO Pat., 2003099195, 2003 Search PubMed.
- R. S. Erdmann, H. Takakura, A. D. Thompson, F. Rivera-Molina, E. S. Allgeyer, J. Bewersdorf, D. Toomre and A. Schepartz, Angew. Chem., 2014, 53, 10242–10246 CrossRef CAS PubMed.
- N. G. Lipsky and R. E. Pagano, Science, 1985, 228, 745–747 CrossRef CAS PubMed.
- G. Wang, M. Dinkins, Q. He, G. Zhu, C. Poirier, A. Campbell, M. Mayer-Proschel and E. Bieberich, J. Biol. Chem., 2012, 287, 21384–21395 CrossRef CAS PubMed.
- B. Obermeier, R. Daneman and R. M. Ransohoff, Nat. Med., 2013, 19, 1584–1596 CrossRef CAS PubMed.
- A. Simonis, S. Hebling, E. Gulbins, S. Schneider-Schaulies and A. Schubert-Unkmeir, PLoS Pathog., 2014, 10, e1004160 CrossRef PubMed.
- A. Unkmeir, K. Latsch, G. Dietrich, E. Wintermeyer, B. Schinke, S. Schwender, K. S. Kim, M. Eigenthaler and M. Frosch, Mol. Microbiol., 2002, 46, 933–946 CrossRef CAS PubMed.
- L. M. Obeid, C. M. Linardic, L. A. Karolak and Y. A. Hannun, Science, 1993, 259, 1769–1771 CrossRef CAS PubMed.
- O. Cuvillier, G. Pirianov, B. Kleuser, P. G. Vanek, O. A. Coso, S. Gutkind and S. Spiegel, Nature, 1996, 381, 800–803 CrossRef CAS PubMed.
- Y. Hirano, S. Yoshinaga, R. Takeya, N. N. Suzuki, M. Horiuchi, M. Kohjima, H. Sumimoto and F. Inagaki, J. Biol. Chem., 2005, 280, 9653–9661 CrossRef CAS PubMed.
- M. Coureuil, G. Mikaty, F. Miller, H. Lecuyer, C. Bernard, S. Bourdoulous, G. Dumenil, R. M. Mege, B. B. Weksler, I. A. Romero, P. O. Couraud and X. Nassif, Science, 2009, 325, 83–87 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc02879a |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |