Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selecting reactions and reactants using a switchable rotaxane organocatalyst with two different active sites†
Jack
Beswick
,
Victor
Blanco
,
Guillaume
De Bo
,
David A.
Leigh
*,
Urszula
Lewandowska
,
Bartosz
Lewandowski
and
Kenji
Mishiro
School of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: david.leigh@manchester.ac.uk; Web: http://www.catenane.net
First published on 13th November 2014
Abstract
The activation mode of a rotaxane-based organocatalyst with both secondary amine and squaramide catalytic units can be switched with acid or base. The macrocycle blocks whichever of the catalytic sites it is positioned over. The switchable rotaxane catalyst generates different products from a mixture of three building blocks according to the location of the macrocyclic ring in the rotaxane.
Introduction
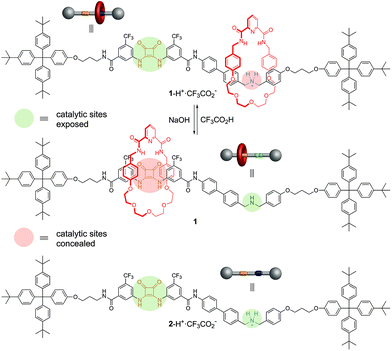
Synthetic catalysts have previously been developed where a stimulus can be used to turn the catalytic activity ‘on’ or ‘off’1,2 or to change the stereochemical outcome of a reaction.3 Here we report on an artificial system that can switch between two different modes of organocatalysis,4 each promoting a different chemical transformation. The result is a molecular catalyst that can be used to produce different reaction outcomes from a mixture of building blocks (Fig. 1).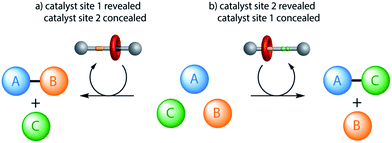 | ||
| Fig. 1 Different products from a mixture of building blocks using a rotaxane catalyst switchable between two different active sites (e.g.1/1-H+·CF3CO2−, Fig. 2). Alternative reactions are promoted (involving particular functional groups on different building blocks) according to which active site of the catalyst is revealed (e.g.Fig. 4). | ||
The switchable catalyst employed is a [2]rotaxane in which the position of the macrocycle can be changed5 to block one or other of two organocatalytic active sites.2,6 The rotaxane (1/1-H+·CF3CO2−, Fig. 2) features a thread bearing dibenzylamine/dibenzylammonium and squaramide units as the catalytic centres. The activities of the organocatalytic sites are based on different activation mechanisms: the secondary amine/ammonium unit is able2 to promote iminium7 (and potentially enamine8 and trienamine9) catalysis while squaramide-catalyzed reactions proceed through the activation of electrophiles by hydrogen bonding.10 The macrocycle of the rotaxane contains a pyridyl-2,6-dicarboxyamide unit that can bind effectively to the squaramide residue, and a crown ether-like region that has a very high affinity for secondary ammonium ions but not for non-protonated amines.11 A rigid spacer was introduced between the two active sites on the thread to prevent folding. Accordingly, when the rotaxane is protonated (1-H+·CF3CO2−) the macrocycle should preferentially encapsulate the dibenzylammonium group, masking it from being available for catalysis (iminium catalysis ‘off’) while leaving the squaramide site accessible (hydrogen bond catalysis ‘on’). In the neutral form of the rotaxane (1) the squaramide should be the preferred binding site for the macrocycle, concealing it and making it unavailable for catalysis (hydrogen bond catalysis ‘off’) whilst leaving the secondary amine exposed (iminium catalysis ‘on’).12
Results and discussion
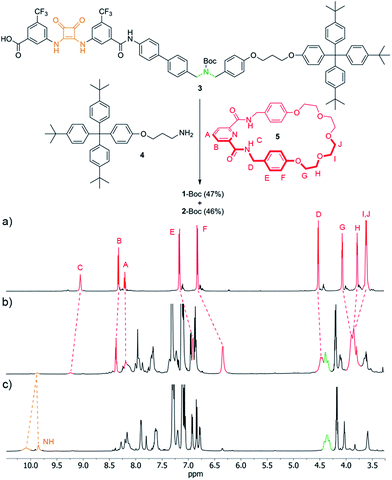
The synthesis of 1 utilized the intended pyridinedicarboxamide-squaramide recognition motif to promote the threading of a suitable squaramide derivative, 3, through the cavity of macrocycle 5, covalently capturing the interlocked structure through amide bond formation with bulky ‘stopper’ 4 (Fig. 3, see ESI† for details). [2]Rotaxane 1-Boc was isolated in 47% yield along with the non-interlocked thread (2-Boc, 46%).The 1H NMR spectra (Fig. 3a–c) of the macrocycle (5), thread (2-Boc) and rotaxane (1-Boc) confirms the threaded architecture of 1-Boc with the macrocycle residing around the squaramide unit. The downfield shift of the HC amide protons in the rotaxane compared to the parent macrocycle (ΔδHC = 0.19 ppm) and the shifts of the protons on the central region of the polyether chain (ΔδHH = 0.08 ppm; ΔδHI,J = 0.25 ppm) indicate hydrogen bonding between the macrocycle and both sides, hydrogen bond donors and acceptors, of the thread squaramide unit. Protons of the phenyl rings of the macrocycle are shifted upfield in the rotaxane (ΔδHE = −0.26 ppm; ΔδHF = −0.48 ppm) due to shielding by the ring currents of the squaramide ring and aryl substituents.
Deprotection of the dibenzylamine moiety using trifluoroacetic acid afforded rotaxane 1-H+·CF3CO2− (see ESI† for details). A solution of 1-H+·CF3CO2− in CH2Cl2 was washed with NaOH(aq) (2 M) to produce 1, 1H NMR spectroscopy confirming the change of position of the macrocycle (see ESI†). Addition of CF3CO2H (1.4 equiv.) to 1 in CH2Cl2 smoothly regenerated 1-H+·CF3CO2− (see ESI†).
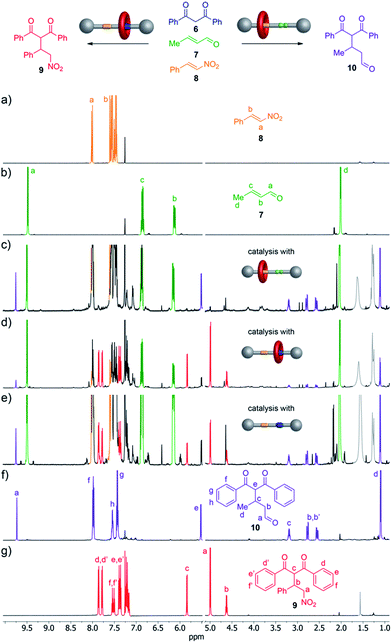
We investigated the ability of the rotaxane and the thread to perform organocatalytic reactions in both their protonated (1-H+·CF3CO2−; 2-H+·CF3CO2−) and unprotonated (1) states. Secondary amines can promote the Michael addition of 1,3-dicarbonyl nucleophiles to α,β-unsaturated aldehydes via iminium catalysis.13 When using a nitroalkene instead of the unsaturated aldehyde a similar Michael addition can occur if the electrophile is activated by hydrogen bond catalysts such as (thio)urea or squaramide derivatives.14 Accordingly, we reasoned that the rotaxane might be able to catalyse the Michael addition of 1,3-diphenylpropane-1,3-dione (6) selectively to either crotonaldehyde (7) or trans-β-nitrostyrene (8) according to which type of organocatalytic group was exposed on the thread.
A mixture of 6 (0.5 M), 7 and 8 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, 10 mol% NaOAc15 and 5 mol% of the potential catalyst (1, 1-H+·CF3CO2− or 2-H+·CF3CO2−) was stirred in CH2Cl2 at room temperature (Fig. 4, top). Rotaxane 1 (secondary amine exposed) catalyzed the Michael addition of 6 to crotonaldehyde (7) to give 10 (40% conversion after 72 h) with high selectivity (only a trace of 9, the addition product to trans-β-nitrostyrene, present in the reaction mixture as evidenced by 1H NMR spectroscopy, Fig. 4c). Use of the protonated form of the rotaxane, 1-H+·CF3CO2−, (squaramide exposed) resulted in the formation of 9 with a conversion of 75% after 18 h with only a few percent of 10 present in the reaction mixture (Fig. 4d).
1 ratio, 10 mol% NaOAc15 and 5 mol% of the potential catalyst (1, 1-H+·CF3CO2− or 2-H+·CF3CO2−) was stirred in CH2Cl2 at room temperature (Fig. 4, top). Rotaxane 1 (secondary amine exposed) catalyzed the Michael addition of 6 to crotonaldehyde (7) to give 10 (40% conversion after 72 h) with high selectivity (only a trace of 9, the addition product to trans-β-nitrostyrene, present in the reaction mixture as evidenced by 1H NMR spectroscopy, Fig. 4c). Use of the protonated form of the rotaxane, 1-H+·CF3CO2−, (squaramide exposed) resulted in the formation of 9 with a conversion of 75% after 18 h with only a few percent of 10 present in the reaction mixture (Fig. 4d).
In contrast to the selectivity found with both forms of the rotaxane catalyst, when the thread 2-H+·CF3CO2− was employed as the catalyst (both organocatalytic sites exposed) 9 and 10 were formed in a close-to-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (15% conversion after 72 h, Fig. 4e).
1 ratio (15% conversion after 72 h, Fig. 4e).
Conclusions
A rotaxane with two different organocatalytic sites, a squaramide unit and a dibenzylamine group, separated by a rigid spacer, has been demonstrated to promote Michael addition reactions through either iminium ion or hydrogen-bond-activated catalysis. The system can be switched between the two activation modes through acid–base-mediated control of the position of the rotaxane macrocycle to conceal one site on the thread and reveal the other. The switchable organocatalyst was used to promote the Michael addition of 1,3-diphenylpropan-1,3-dione (6) to either crotonaldehyde (7) or trans-β-nitrostyrene (8) according to the catalyst state, with modest conversions (40–75%) and good selectivity in both modes.The ability to select which components of a mixture react together, affording different product outcomes from a common set of building blocks, is a promising use of artificial molecular machines in chemical synthesis.16
Acknowledgements
This research was funded by the EPSRC. We are grateful to the following organizations for postdoctoral fellowships: Fundacja na Rzecz Nauki Polskiej (to B.L.), Fonds Spécial de Recherche – Fédération Wallonie-Bruxelles and Wallonie-Bruxelles International (to G.D.B.), and the European Union 7th Framework Marie Curie Intra-European Fellowship Programme (to V.B.).Notes and references
- For examples of catalysts that can be switched ‘on’ and ‘off’ by a specific stimulus, see: F. Würthner and J. Rebek Jr, Angew. Chem., Int. Ed. Engl., 1995, 34, 446 CrossRef CAS; H. J. Yoon, J. Kuwabara, J.-H. Kim and C. A. Mirkin, Science, 2010, 330, 66 CrossRef PubMed; Y. Sohtome, S. Tanaka, K. Takada, T. Yamaguchi and K. Nagasawa, Angew. Chem., Int. Ed., 2010, 49, 9254 CrossRef PubMed; J. Berná, M. Alajarín and R.-A. Orenes, J. Am. Chem. Soc., 2010, 132, 10741 CrossRef PubMed; M. Zirngast, E. Pump, A. Leitgeb, J. H. Albering and C. Slugovc, Chem. Commun., 2011, 47, 2261 RSC; O. B. Berryman, A. C. Sather, A. Lledó and J. Rebek Jr, Angew. Chem., Int. Ed., 2011, 50, 9400 CrossRef PubMed; U. Lüning, Angew. Chem., Int. Ed., 2012, 51, 8163 CrossRef PubMed; B. M. Neilson and C. W. Bielawski, J. Am. Chem. Soc., 2012, 134, 12693 CrossRef PubMed; M. Schmittel, S. De and S. Pramanik, Angew. Chem., Int. Ed., 2012, 51, 3832 CrossRef PubMed; M. Schmittel, S. Pramanik and S. De, Chem. Commun., 2012, 48, 11730 RSC; P. Viehmann and S. Hecht, Beilstein J. Org. Chem., 2012, 8, 1825 CrossRef PubMed; D. Wilson and N. R. Branda, Angew. Chem., Int. Ed., 2012, 51, 5431 CrossRef PubMed; B. M. Neilson and C. W. Bielawski, Chem. Commun., 2013, 49, 5453 RSC; B. M. Neilson and C. W. Bielawski, Organometallics, 2013, 32, 3121 CrossRef; B. M. Neilson and C. W. Bielawski, ACS Catal., 2013, 3, 1874 CrossRef; L. Osorio-Planes, C. Rodríguez-Escrich and M. A. Pericás, Org. Lett., 2014, 16, 1704 CrossRef PubMed; C. M. McGuirk, C. L. Stern and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 4689 CrossRef PubMed.
- For rotaxane-based secondary amine organocatalysts whose efficacy can be turned ‘on’ and ‘off’ by acid–base switching of the position of the macrocycle, see: V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, Angew. Chem., Int. Ed., 2012, 51, 5166 CrossRef CAS PubMed; V. Blanco, D. A. Leigh, V. Marcos, J. A. Morales-Serna and A. L. Nussbaumer, J. Am. Chem. Soc., 2014, 136, 4905 CrossRef PubMed; V. Blanco, D. A. Leigh, U. Lewandowska, B. Lewandowski and V. Marcos, J. Am. Chem. Soc., 2014, 136, 15775 CrossRef PubMed.
- J. Wang and B. L. Feringa, Science, 2011, 331, 1429 CrossRef CAS PubMed; S. Mortezaei, N. R. Catarineu and J. W. Canary, J. Am. Chem. Soc., 2012, 134, 8054 CrossRef PubMed; M. Vlatkovic, L. Bernardi, E. Otten and B. L. Feringa, Chem. Commun., 2014, 50, 7773 RSC.
- G. Lelais and D. W. C. MacMillan, Aldrichimica Acta, 2006, 39, 79 Search PubMed; A. Erkkilä, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef CAS PubMed; P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef PubMed; S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC.
- E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 CrossRef CAS PubMed For examples of rotaxane-based molecular machines performing other useful tasks, see: C. P. Collier, E. W. Wong, M. Belohradský, F. M. Raymo, J. F. Stoddart, P. J. Kuekes, R. S. Williams and J. R. Heath, Science, 1999, 285, 391 CrossRef; E. M. Pérez, D. T. F. Dryden, D. A. Leigh, G. Teobaldi and F. Zerbetto, J. Am. Chem. Soc., 2004, 126, 12210 CrossRef PubMed; Q.-C. Wang, D.-H. Qu, J. Ren, K. Chen and H. Tian, Angew. Chem., Int. Ed., 2004, 43, 2661 CrossRef; D. A. Leigh, M. Á. F. Morales, E. M. Pérez, J. K. Y. Wong, C. G. Saiz, A. M. Z. Slawin, A. J. Carmichael, D. M. Haddleton, A. M. Brouwer, W. J. Buma, G. W. H. Wurpel, S. León and F. Zerbetto, Angew. Chem., Int. Ed., 2005, 44, 3062 CrossRef PubMed; J. Berná, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Pérez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704 CrossRef PubMed; T. D. Nguyen, H.-R. Tseng, P. C. Celestre, A. H. Flood, Y. Liu, J. F. Stoddart and J. I. Zink, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10029 CrossRef PubMed; Y.-L. Huang, W.-C. Hung, C.-C. Lai, Y.-H. Liu, S.-M. Peng and S.-H. Chiu, Angew. Chem., Int. Ed., 2007, 46, 6629 CrossRef PubMed; W. Zhou, J. Li, X. He, C. Li, J. Lv, Y. Li, S. Wang, H. Liu and D. Zhu, Chem.–Eur. J., 2008, 14, 754 CrossRef PubMed; A. Fernandes, A. Viterisi, F. Coutrot, S. Potok, D. A. Leigh, V. Aucagne and S. Papot, Angew. Chem., Int. Ed., 2009, 48, 6443 CrossRef PubMed; J. J. Gassensmith, S. Matthys, J.-J. Lee, A. Wojcik, P. Kamat and B. D. Smith, Chem.–Eur. J., 2010, 16, 2916 CrossRef PubMed; P. Lussis, T. Svaldo-Lanero, A. Bertocco, C.-A. Fustin, D. A. Leigh and A.-S. Duwez, Nat. Nanotechnol., 2011, 6, 553 CrossRef PubMed; A. V. Leontiev, C. A. Jemmett and P. D. Beer, Chem.–Eur. J., 2011, 17, 816 CrossRef PubMed; C. J. Serpell, R. Chall, A. L. Thompson and P. D. Beer, Dalton Trans., 2011, 40, 12052 RSC; A. Fernandes, A. Viterisi, V. Aucagne, D. A. Leigh and S. Papot, Chem. Commun., 2012, 48, 2083 RSC; T. Avellini, H. Li, A. Coskun, G. Barin, A. Trabolsi, A. N. Basuray, S. K. Dey, A. Credi, S. Silvi, J. F. Stoddart and M. Venturi, Angew. Chem., Int. Ed., 2012, 51, 1611 CrossRef PubMed.
- For rotaxanes incorporating catalytic centers, see: P. Thordarson, E. J. Bijsterveld, A. E. Rowan and R. J. Nolte, Nature, 2003, 424, 915 CrossRef CAS PubMed; Y. Tachibana, N. Kihara and T. Takata, J. Am. Chem. Soc., 2004, 126, 3438 CrossRef PubMed; G. Hattori, T. Hori, Y. Miyake and Y. Nishibayashi, J. Am. Chem. Soc., 2007, 129, 12930 CrossRef PubMed; Y. Li, Y. Feng, Y.-M. He, F. Chen, J. Pan and Q.-H. Fan, Tetrahedron Lett., 2008, 49, 2878 CrossRef PubMed; N. Miyagawa, M. Watanabe, T. Matsuyama, Y. Koyama, T. Moriuchi, T. Hirao, Y. Furusho and T. Takata, Chem. Commun., 2010, 46, 1920 RSC; Y. Suzaki, K. Shimada, E. Chihara, T. Saito, Y. Tsuchido and K. Osakada, Org. Lett., 2011, 13, 3774 CrossRef PubMed; D. A. Leigh, V. Marcos and M. R. Wilson, ACS Catal., 2014, 4, 4490 CrossRef.
- S. B. Tsogoeva, Eur. J. Org. Chem., 2007, 1701 CrossRef CAS; D. Almasi, D. A. Alonso and C. Nájera, Tetrahedron: Asymmetry, 2007, 18, 299 CrossRef PubMed; J. L. Vicario, D. Badía and L. Carrillo, Synthesis, 2007, 2065 CrossRef PubMed; A. Erkkilä, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef PubMed; M. Shimizu, I. Hachiya and I. Mizota, Chem. Commun., 2009, 874 RSC.
- W. Notz, F. Tanaka and C. F. Barbas III, Acc. Chem. Res., 2004, 37, 580 CrossRef CAS PubMed; S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef PubMed; G. Guillena, C. Nájera and D. J. Ramon, Tetrahedron: Asymmetry, 2007, 18, 2249 CrossRef PubMed; T. Kano and K. Maruoka, Chem. Sci., 2013, 4, 907 RSC; D. Deng, S. Kumar and H. Wang, Chem. Commun., 2014, 50, 4272 RSC; A. Desmarchelier, V. Coeffard, X. Moreau and C. Greck, Tetrahedron, 2014, 70, 2491 CrossRef PubMed.
- H. Jiang, Ł. Albrecht and K. A. Jørgensen, Chem. Sci., 2013, 4, 2287 RSC; I. Kumar, P. Ramaraju and N. A. Mir, Org. Biomol. Chem., 2013, 11, 709 Search PubMed; I. D. Jurberg, I. Chatterjee, R. Tanert and P. Melchiorre, Chem. Commun., 2013, 49, 4869 RSC.
- J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed; Y. Zhu, J. P. Malerich and V. H. Rawal, Angew. Chem., Int. Ed., 2010, 49, 153 CrossRef PubMed; J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2011, 17, 6890 CrossRef PubMed.
- D. A. Leigh and A. R. Thomson, Org. Lett., 2006, 8, 5377 CrossRef CAS PubMed For other host–guest complexes and rotaxanes that employ this or similar motifs, see: L. Huang, W.-C. Hung, C.-C. Lai, Y.-H. Liu, S.-M. Peng and S.-H. Chiu, Angew. Chem., Int. Ed., 2007, 46, 6629 CrossRef PubMed; S.-Y. Hsueh, C.-T. Kuo, T.-W. Lu, C.-C. Lai, Y.-H. Liu, H.-F. Hsu, S.-M. Peng, C.-h. Chen and S.-H. Chiu, Angew. Chem., Int. Ed., 2010, 49, 9170 CrossRef PubMed; L. Liu, Y. Liu, P. Liu, J. Wu, Y. Guan, X. Hu, C. Lin, Y. Yang, X. Sun, J. Ma and L. Wang, Chem. Sci., 2013, 4, 1701 RSC.
- The acid–base switching of the position of a related macrocycle between dibenzylamine/ammonium motifs and amide-based functional groups in a rotaxane has previously been described (ref. 11).
- S. Brandau, A. Landa, J. Franzén, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 4305 CrossRef CAS PubMed; A. Carlone, M. Marigo, C. North, A. Landa and K. A. Jørgensen, Chem. Commun., 2006, 4928 RSC; A. Carlone, S. Cabrera, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2007, 46, 1101 CrossRef PubMed; S. P. Lathrop and T. Rovis, J. Am. Chem. Soc., 2009, 131, 13628 CrossRef PubMed.
- T. Okino, Y. Hoashi, T. Furukawa, X. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119 CrossRef CAS PubMed; J. Wang, H. Li, W. Duan, L. Zu and W. Wang, Org. Lett., 2005, 7, 4713 CrossRef PubMed; S. J. Connon, Chem.–Eur. J., 2006, 15, 5418 CrossRef PubMed; Z.-H. Zhang, X.-Q. Dong, D. Chen and C.-J. Wang, Chem.–Eur. J., 2008, 14, 8780 CrossRef PubMed; Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187 RSC; R. I. Storer, C. Aciro and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330 RSC.
- Sodium acetate is used as a base to activate the nucleophile.
- B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189 CrossRef CAS PubMed; G. De Bo, S. Kuschel, D. A. Leigh, B. Lewandowski, M. Papmeyer and J. W. Ward, J. Am. Chem. Soc., 2014, 136, 5811 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterisation data. See DOI: 10.1039/c4sc03279a |
| This journal is © The Royal Society of Chemistry 2015 |