Synthesis of amphiphilic fluorescent PEGylated AIE nanoparticles via RAFT polymerization and their cell imaging applications
Zengfang Huang*ab,
Xiqi Zhangbc,
Xiaoyong Zhangb,
Shiqi Wangb,
Bin Yangb,
Ke Wangb,
Jinying Yuanb,
Lei Tao*b and
Yen Wei*b
aCollege of Chemistry and Biology, Zhongshan Institute, University of Electronic Science & Technology of China, Zhongshan, 528402, P. R. China. E-mail: hzf105@163.com
bDepartment of Chemistry, the Tsinghua Center for Frontier Polymer Research, Tsinghua University, Beijing 100084, P. R. China. E-mail: leitao@mail.tsinghua.edu.cn; weiyen@tsinghua.edu.cn
cLaboratory of Bio-Inspired Smart Interface Science, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 5th October 2015
Abstract
With the increasing interest in the use of luminescent probes in biomedical applications, the development of fluorescent organic nanoparticles (FONs) on the basis of aggregation induced emission (AIE) dyes has attracted great research attention. In this study, a polymerizable tetraphenylethene-functionalized AIE dye (named as TPEV) with a vinyl end functional group was synthesized by a “one-step” Suzuki coupling reaction of 4-vinylphenylboronic acid and bromotriphenylethylene, and the as-prepared hydrophobic AIE dye TPEV subsequently participated in the reversible addition–fragmentation chain transfer (RAFT) polymerization together with the hydrophilic monomer of poly(ethylene glycol) monomethacrylate (PEGMA) to obtain a new amphiphilic copolymer (denoted as TPEV–PEG) with transformed side fluorescent groups. The Mn value of the obtained copolymer was about 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 with a narrow polydispersity index (PDI) of about 1.30. The molar ratio of TPE to PEG segment in the copolymer was respectively about 19.2% to 80.8%, and it was easy for the TPEV–PEG copolymer to self-assemble into FONs with the hydrophobic AIE core encapsulated by a hydrophilic PEG shell. The research results further showed that the TPEV–PEG FONs presented good fluorescent features with the maximal emission peak at 480 nm, high dispersibility in water solution with homogeneous spherical morphology (∼200 nm) and excellent biocompatibility, giving them good potential for bioimaging applications.
800 g mol−1 with a narrow polydispersity index (PDI) of about 1.30. The molar ratio of TPE to PEG segment in the copolymer was respectively about 19.2% to 80.8%, and it was easy for the TPEV–PEG copolymer to self-assemble into FONs with the hydrophobic AIE core encapsulated by a hydrophilic PEG shell. The research results further showed that the TPEV–PEG FONs presented good fluorescent features with the maximal emission peak at 480 nm, high dispersibility in water solution with homogeneous spherical morphology (∼200 nm) and excellent biocompatibility, giving them good potential for bioimaging applications.
1. Introduction
As a metal-free approach, RAFT polymerization has excellent tolerance to many functional monomers and numerous solvents, and is regarded as a powerful tool to facilely fabricate numerous functional polymers with expectant molecular weights and architectures with narrow polydispersity index (PDI).1–7 Recently, our group reported a one-pot combination of RAFT polymerization and transesterification of 2,2,2-trifluoethyl methacrylate (TFEMA) with n-hexanol, and the kinetics indicated that the molecular weight increased linearly with monomer conversion and the polymerization had the characteristics of a controllable polymerization.8 Another novel ‘one pot’ KF-RAFT strategy was successfully developed by combining an effective KF reaction and RAFT process, and this system is facile and efficient for the preparation of new side functionalized poly(aminophosphonate)s (polyAPPs).9 Moreover, the RAFT polymerization method was also adopted to prepare AIE copolymers. Taking EDMAT and AIBN as the free chain transfer agent and the radical initiator, an AIE copolymer of styrene or 4-vinylpyridine was successfully prepared, subsequently, the block amphiphilic AIE copolymer was further obtained by the reaction of polyvinylpyridine copolymer with an excess amount of iodomethane in DMSO.10 Red cross-linkable R-PEG FONs via RAFT polymerization of a cross-linkable AIE dye (R-E) and PEGMA were reported in our previous work.11 Without catalysts or initiators, a novel technique for preparing PhNH2–OA–PEG FONs has been further investigated via a one-pot combination of ring-opening polymerization and condensation reaction at room temperature and in air.12,13 These as-prepared FONs showed strong fluorescence, high water dispersibility, and excellent biocompatibility, which makes them promising for applications in the bioimaging field.Recently, the development of FONs based on AIE dyes has attracted great research attention.14 Due to scientists’ enthusiastic investigation, a large variety of AIE molecules have been developed with great structural variety, and a few typical examples are tetraphenylethene (TPE), hexaphenylsilole (HPS) and distyreneanthracene (DSA).15–17 Among these AIE molecules, the TPE derivative is a class of widely known AIE material, which has been expansively developed for chemosensor and biomedical applications.18–20 Although there has been great progress with small molecular AIE dyes, polymers and other macromolecules as the basis of AIE molecules have been less explored.21 As compared with small molecules, polymer materials have many advantages, such as various opportunities to adjust the structure, topology and morphology, as well as easy functionalization.22 Therefore, polymerization with an AIE dye provides a much larger platform for material fabrication. For example, a poly(N-isopropylacrylamide) (PNIPAM) with a TPE fluorogenic segment was acquired by direct copolymerization of N-isopropylacrylamide and a monomer containing a TPE segment via AIBN-initiated free radical polymerization. Thus, it is effective for the incorporation of a small amount of AIE materials to convert PNIPAM into an AIE polymer while keeping the original characteristics of PNIPAM.23 In other work, the partially substituted polymer of poly(acrylic acid) (PAA) was obtained via amidation of an amino-containing TPE derivative linked onto PAA. The acid functional groups and hydrophobic TPE pendants respectively made the polymer have pH-responsiveness and AIE features.17 The linear polymer with TPE building blocks was facilely generated by Suzuki coupling polymerization of TPE-containing dibromide and diboronic acid, and the as-prepared polymer had high thermal stability and an AIE-active feature from the rigid structure and TPE segment.24 TPE-containing diacrylates were polymerized using AIBN as an initiator in refluxing THF under mild reaction conditions, and the addition of a small amount of water into the THF solution causes an obvious increase of the fluorescence of the obtained polymer.25 Our group facilely prepared TPE-based AIE FONs with stable C–N covalent bonds via Schiff base condensation with ε-polylysine (Ply).26 Another one-pot strategy for the fabrication of TPE-based FONs was developed via combination of a RAFT polymerization and a transesterification reaction, and the molar fraction of TPE and PEG in the polymer was about 30.5% and 69.5%. These FONs presented spherical morphology, uniform size, and excellent biocompatibility.27
It is a powerful and convenient strategy to use RAFT polymerization of a polymerizable AIE monomer combined with some hydrophilic monomer to fabricate the amphiphilic AIE copolymer, which will tend to self-assemble into FONs, making them promising for bioimaging applications. In this contribution, a polymerizable AIE dye of tetraphenylethene-functionalized monomer (TPEV) was synthesized by a Suzuki coupling reaction of 4-vinylphenylboronic acid and bromotriphenylethylene, which subsequently participated in the RAFT polymerization with the hydrophilic monomer of PEGMA to obtain a new amphiphilic AIE copolymer (TPEV–PEG) with transformed side fluorescent groups. The obtained TPEV–PEG copolymer tended to self-assemble into FONs in aqueous solution. To study the cell imaging application, the dispersibility, AIE property, and biocompatibility of TPEV–PEG FONs were further investigated.
2. Experimental
2.1. Materials and characterization
Poly(ethylene glycol) monomethacrylate (PEGMA, Mn = 500, J&K Chemical, AR), 2,2′-azobisisoheptonitrile (AVBN, J&K Chemical, 98%), tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, J&K Chemical, 97%), 4-vinylphenylboronic acid (J&K Chemical, 97%), bromotriphenylethylene (J&K Chemical, 95%), tetrabutyl ammonium bromide (TBAB, J&K Chemical, 98%), and triethylamine (TEA, J&K Chemical, AR) were all used as purchased. The chain transfer agent (CTA) of 4-cyano-4-(ethylthiocarbonothioylthio) pentanoic acid was synthesized by reference to the literature methods.28Gel permeation chromatography (GPC) analysis of the TPEV–PEG copolymer was performed on a CBM-20A at room temperature based on standard polystyrene as the reference with N,N-dimethyl formamide (DMF) as the solvent. 1H NMR spectra of TPEV dye and its TPEV–PEG copolymer were carried out on a JEOL JNM-ECA 400 (400 MHz) spectrometer at room temperature in a CDCl3 solution with tetramethylsilane (TMS) as a reference. Elemental analysis (EA) of TPEV dye was performed on an Elementar Vario EL elemental analyzer. The transmission electron microscopy (TEM) specimen was made by placing a drop of the TPEV–PEG suspension on a carbon-coated copper grid, and the TEM image was recorded on a JEM-1200EX microscope operated at 100 kV. The UV-vis absorption spectrum of TPEV–PEG copolymer was performed on a Perkin-Elmer LAMBDA 35 UV-vis system. Fluorescence emission (FL) spectra of TPEV–PEG in water or THF solution were measured on a PE LS-55 spectrometer. The FT-IR spectra of TPEV dye and TPEV–PEG copolymer were obtained in reflection mode on a Perkin-Elmer Spectrum 100 spectrometer (Waltham, MA, USA).
2.2. Synthesis of tetraphenylethene-functionalized vinyl (TPEV)
The synthesis of the fluorescent TPEV dye was as follows: bromotriphenylethylene (1.05 g, 3.13 mmol) and 4-vinylphenylboronic acid (0.56 g, 3.75 mmol) were dissolved in the mixture of toluene (22 mL), TBAB (0.10 g, 0.31 mmol) and 2 M potassium carbonate aqueous solution (5.6 mL). The mixture was stirred at room temperature for 0.5 h under N2 gas followed by the addition of Pd(PPh3)4 (4.2 mg, 3.65 × 10−3 mmol) and then heated to 90 °C for 24 h. Subsequently, the mixture was poured into water and extracted three times with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate. After removing the solvent under vacuum, the residue was chromatographed on a silica gel column with n-hexane–CH2Cl2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) as eluent to give TPEV (0.85 g, 76% yield). 1H NMR (400 MHz, CDCl3, δ): 5.17 (d, J = 8.0 Hz, 1H; CH), 5.66 (d, J = 12.0 Hz, 1H; CH), 6.61 (t, J = 8.0 Hz, 1H; CH), 6.96–7.15 (m, 19H; Ar H); 13C NMR (100 MHz, CDCl3, δ): 113.56, 125.62, 126.50, 126.56, 127.71, 127.84, 131.43, 131.62, 135.58, 136.66, 140.65, 141.12, 143.43, 143.79; anal. calcd for C28H22: C 93.81, H 6.19; found: C 93.69, H 6.31.
1 by volume) as eluent to give TPEV (0.85 g, 76% yield). 1H NMR (400 MHz, CDCl3, δ): 5.17 (d, J = 8.0 Hz, 1H; CH), 5.66 (d, J = 12.0 Hz, 1H; CH), 6.61 (t, J = 8.0 Hz, 1H; CH), 6.96–7.15 (m, 19H; Ar H); 13C NMR (100 MHz, CDCl3, δ): 113.56, 125.62, 126.50, 126.56, 127.71, 127.84, 131.43, 131.62, 135.58, 136.66, 140.65, 141.12, 143.43, 143.79; anal. calcd for C28H22: C 93.81, H 6.19; found: C 93.69, H 6.31.
2.3. Synthesis of fluorescent copolymer (TPEV–PEG)
The fabrication of the fluorescent copolymer TPEV–PEG was as follows: TPEV (50 mg, 0.140 mmol), PEGMA (310 mg, 0.620 mmol), CTA (2.8 mg, 1.06 × 10−2 mmol), AVBN (1.3 mg, 0.524 × 10−2 mmol) and 1.0 mL toluene solvent were added into a Schlenk tube equipped with a magnetic stir bar, and then subjected to a freeze–pump–thaw cycle with nitrogen three times. The Schlenk tube was then introduced into an oil bath kept at 55 °C for 30 h. Finally, the mixed solvent was removed under vacuum. The copolymer was further purified by precipitation from THF to petroleum ether three times, and then dried under vacuum for characterization and cell imaging applications. Yield: 0.30 g. Finally, 10 mg TPEV–PEG copolymer was added to 5 mL H2O, then shaken until it had been dissolved completely, which was used to investigated its self-assembly in H2O solution.2.4. Cytotoxicity of TPEV–PEG FONs
The observation of cell morphology was used to investigate the effects of TPEV–PEG FONs on HepG2 cells.29 Briefly, cells were seeded into 6-well microplates in 2 mL of respective media at a density of 1 × 105 cells per mL containing 10% fetal bovine serum (FBS). After cell attachment, plates were washed with PBS and cells were treated with complete cell culture medium, or different concentrations of TPEV–PEG FONs prepared in 10% FBS containing media for 24 h. Then all samples were washed with PBS three times to remove the uninternalized FONs. An optical microscope (Leica, Germany) was used to observe the morphology of cells, whose overall magnification was ×100.The cell counting kit-8 (CCK-8) assay was used to examine the cell viability of TPEV–PEG FONs on HepG2 cells on the basis of our previous reports. In brief, cells were seeded in 96-well microplates at a density of 5 × 104 cells per mL in 160 μL of the respective media containing 10% FBS. After cell attachment for 24 h, the cells were incubated with 10, 20, 40, 80, 120 μg mL−1 TPEV–PEG for 8 and 24 h, and then the cells were washed with PBS three times to remove the uninternalized TPEV–PEG FONs. 10 μL of CCK-8 dye and 100 μL of DMEM cell culture medium were added into each well and incubated for 2 h at 37 °C. Finally, the plates were analyzed with a microplate reader (Victor III, Perkin-Elmer). The absorbance of formazan dye was obtained at 450 nm, with 620 nm as the reference wavelength. The absorbance values were proportional to the number of live cells. The percent reduction of CCK-8 dye was obtained taking controls (cells not exposure to TPEV–PEG FONs) as the reference, which represented 100% CCK-8 reduction. The microplate experiment was repeated three times with three replicate wells. Cell survival was expressed as absorbance relative to that of untreated controls, and the results are presented as mean ± standard deviation (SD).
2.5. Confocal microscopic imaging of cells using TPEV–PEG FONs
The cell uptake of TPEV–PEG FONs was further evaluated by confocal microscopic imaging.27,30 Briefly, cells were seeded in a glass bottomed dish with a density of 1 × 105 cells per dish. On the day of treatment, the cells were incubated with TPEV–PEG FONs at a final concentration of 80 μg mL−1 for 3 h at 37 °C. Afterwards, the cells were washed three times with PBS to remove the TPEV–PEG FONs and then fixed with 4% paraformaldehyde for 10 min at room temperature. Cell images were obtained by a confocal laser scanning microscope Zeiss 710 3-channels (Zeiss, Germany) with 405 nm as the excitation wavelength.3. Results and discussion
Previously, a polymerizable tetraphenylethene-functionalized AIE dye was successfully synthesized by a “three-steps” method: synthesis of 1-(4-bromophenyl)-1,2,2-triphenylethylene (1), converting bromine into formyl group in (1) to product compound (2) and the Wittig reaction of (2) affording vinyl tetraphenylethylene dye.31 Herein, the TPEV dye was synthesized by a “one-step” Suzuki coupling reaction of 4-vinylphenylboronic acid and bromotriphenylethylene with high yield instead of the above “three-steps” method,18 which subsequently copolymerized with the hydrophilic monomer of PEGMA to produce new amphiphilic fluorescent copolymers with transformed side fluorescent groups via RAFT polymerization. The obtained TPEV–PEG copolymers should have excellent fluorescence by the introduction of the TPEV dye; moreover, the hydrophilic PEG chain should also endow them with the good water solubility. It was expected that the obtained amphiphilic fluorescent copolymers would be self-assembled into nanoparticles and further internalized by cells. The synthetic procedure in this report is schematically displayed in Scheme 1.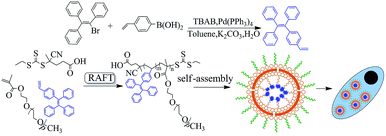 | ||
| Scheme 1 Schematic showing the synthesis of TPEV fluorescent dye and its TPEV–PEG copolymer through RAFT polymerization, and then self-assembly of these copolymers for cell imaging. | ||
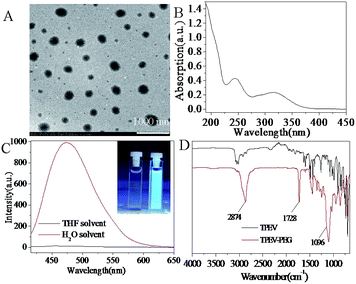
Fig. 1A shows the number average molecular weight (Mn) and the PDI of the final obtained TPEV–PEG copolymers, which were respectively about 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 and 1.30. The structures of the TPEV dye and TPEV–PEG copolymer were analyzed by 1H NMR spectra as exhibited in Fig. 1B. For the TPEV spectrum, the phenyl hydrogen peaks of TPEV appear clearly in the range of 6.96–7.25 ppm, and the characteristic peaks of the polymerizable CH2
800 g mol−1 and 1.30. The structures of the TPEV dye and TPEV–PEG copolymer were analyzed by 1H NMR spectra as exhibited in Fig. 1B. For the TPEV spectrum, the phenyl hydrogen peaks of TPEV appear clearly in the range of 6.96–7.25 ppm, and the characteristic peaks of the polymerizable CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– group can be clearly observed at 5.17, 5.66, and 6.61 ppm, the integral ratio of which is 1
CH– group can be clearly observed at 5.17, 5.66, and 6.61 ppm, the integral ratio of which is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, confirming the successful synthesis of the TPEV dye. For the TPEV–PEG copolymer spectrum, the characteristic peaks of the CH2
1, confirming the successful synthesis of the TPEV dye. For the TPEV–PEG copolymer spectrum, the characteristic peaks of the CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– group have disappeared, and the phenyl hydrogen peaks of the TPEV segment are clearly observed in the range of 6.96–7.10 ppm with the characteristic peak of the ester group linked to the acrylate of the PEGMA at 4.05 ppm,27 and the peaks at 3.63 ppm and 3.36 ppm should be the other ester groups of the PEG segment and –CH3 at the PEGMA end, indicating the successful incorporation of both TPEV and PEGMA into the copolymers via RAFT polymerization. Referring to the integral area ratio of the peaks at 6.96–7.10 ppm and 4.05 ppm, the respective molar fraction of TPEV segment and PEG segment in the copolymers was calculated to be about 19.2% and 80.8%.
CH– group have disappeared, and the phenyl hydrogen peaks of the TPEV segment are clearly observed in the range of 6.96–7.10 ppm with the characteristic peak of the ester group linked to the acrylate of the PEGMA at 4.05 ppm,27 and the peaks at 3.63 ppm and 3.36 ppm should be the other ester groups of the PEG segment and –CH3 at the PEGMA end, indicating the successful incorporation of both TPEV and PEGMA into the copolymers via RAFT polymerization. Referring to the integral area ratio of the peaks at 6.96–7.10 ppm and 4.05 ppm, the respective molar fraction of TPEV segment and PEG segment in the copolymers was calculated to be about 19.2% and 80.8%.
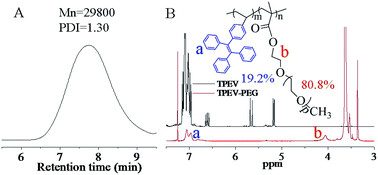 | ||
| Fig. 1 (A) The GPC trace of TPEV–PEG (in DMF); (B) 1H NMR spectra (CDCl3) of TPEV fluorescent dye (black line) and the final obtained fluorescent copolymer TPEV–PEG (red line). | ||
The characterization information including TEM, UV, FL and FT-IR of the prepared TPEV dye and TPEV–PEG FONs are shown in Fig. 2. Transmission electron microscopy (TEM) of Fig. 2A indicates that TPEV–PEG copolymers tend to self-assemble into uniform spherical morphology of about 200 nm, which was further confirmed by DLS, the results of which also indicated that the size was about 200 nm. The spherical morphology by self-assembly of the copolymers further confirms the successful incorporation of TPEV dye and PEGMA into the TPEV–PEG copolymers via RAFT polymerization. The UV absorption spectrum of a water solution of TPEV–PEG FONs is displayed in Fig. 2B, and the absorption peaks are present at 245 nm and 316 nm, which might be caused by the electron transition of π → π*. The light transmission of the nanoparticles will be effectively reduced by the light scattering or Mie effect, resulting in the apparent high absorption and levelling-off of the tail in the visible region.32 It is clear that no absorption in the entire spectrum is discovered until the wavelength is below 390 nm, which is different from our previous result,33 indicating the excellent water dispersibility of TPEV–PEG copolymers. The surface of the TPEV–PEG FONs is encapsulated by hydrophilic PEG to form a PEGylated AIE-based structure, which will endow the TPEV–PEG copolymers with amphiphilic properties, making them tend to self-assemble into nanoparticles with excellent water dispersibility. Fig. 2C demonstrates the fluorescence property of the obtained TPEV–PEG copolymers. From the fluorescence curve, a maximal emission peak at 480 nm is observed in the water solution, but almost no fluorescence is observed in the THF solution, implying an obvious AIE property. The hydrophobic TPEV AIE dye cannot dissolve in water but can in some organic solvents, so the obtained TPEV–PEG copolymers have better dissolution in some organic solvents than in water as solvent. It is common for some compounds to show the AIE phenomenon, but the reason still remains unclear. A possible explanation for the AIE phenomenon is that the solute will aggregate into two kinds of nanoparticle suspensions: crystal particles and amorphous particles. The former will enhance the fluorescent intensity with a shorter emission wavelength, but the latter will reduce the intensity with a longer emission wavelength. The TPEV dye in the copolymers will exist as a more crystalline state in water solution but a more amorphous state in THF solution.32,34 Another possible explanation is that the intramolecular rotation is active in some organic solutions, serving as a relaxation avenue for the excited state to decay, while the rotation is restricted in the aggregation state due to the physical constraint, which blocks the non-radiative path and activates the radiative decay.35
The FT-IR spectra of TPEV dye and TPEV–PEG copolymer are further described as shown in Fig. 2D. For the TPEV spectrum, the stretching vibration of the polycyclic aromatic rings locates in the range of 1380 to 1540 cm−1 with a series of absorbance peaks, and the peaks at 3020–3070 cm−1 can be assigned to the C–H stretching vibration of polycyclic aromatics. Moreover, two characteristic peaks located at 1600 and 1630 cm−1 are caused by the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and CH2
C and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– bonds. For the spectrum of TPEV–PEG copolymer, the peak of –CH2–, –CH3 and polycyclic aromatic rings can also be clearly observed at 2874 cm−1 and the characteristic peak of the C
CH– bonds. For the spectrum of TPEV–PEG copolymer, the peak of –CH2–, –CH3 and polycyclic aromatic rings can also be clearly observed at 2874 cm−1 and the characteristic peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak is observed at 1740 cm−1. As compared with the TPEV spectrum, the peak located at 1630 cm−1 of the CH2
O stretching vibration peak is observed at 1740 cm−1. As compared with the TPEV spectrum, the peak located at 1630 cm−1 of the CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– bond has almost disappeared, indicating the successful incorporation of TPEV dye into the TPEV–PEG copolymer via RAFT polymerization. Otherwise, the spectrum presents one characteristic peak of the C–O stretching vibration at 1100 cm−1, which also confirms the successful incorporation of PEGMA into the copolymer by RAFT polymerization.
CH– bond has almost disappeared, indicating the successful incorporation of TPEV dye into the TPEV–PEG copolymer via RAFT polymerization. Otherwise, the spectrum presents one characteristic peak of the C–O stretching vibration at 1100 cm−1, which also confirms the successful incorporation of PEGMA into the copolymer by RAFT polymerization.
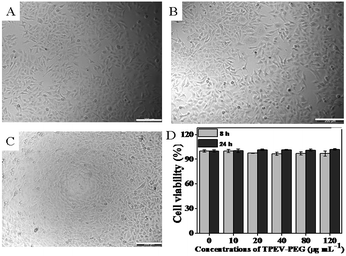
The observation of cell morphology was used to investigate the biocompatibility of TPEV–PEG FONs with HepG2 cells when they were incubated with different concentrations of TPEV–PEG FONs for 24 h as shown in Fig. 3,36–39 and the cell viability of TPEV–PEG FONs on HepG2 cells using the cell counting kit-8 (CCK-8) assay was also examined through the absorbance value of formazan dye at 450 nm with 620 nm as the reference wavelengths.40,41 Optical microscopy observations (Fig. 3A–C) indicated that cells maintained their normal morphology after they were incubated with different concentrations of TPEV–PEG FONs. No obvious cell morphology change could be observed even when the concentration of TPEV–PEG FONs was increased to 80 mg mL−1. To further confirm the good biocompatibility of TPEV–PEG FONs, the cell viability of TPEV–PEG FONs with HepG2 cells was determined using the cell counting kit-8 (CCK-8) assay as shown in Fig. 3D,42–44 and the results demonstrated that no obvious decrease of cell viability was observed when the cells were incubated with 10–120 mg mL−1 of TPEV–PEG FONs. Even when the concentration was as high as 120 mg mL−1, the cell viability values were still more than 90%. From the above results, it was confirmed that TPEV–PEG FONs had good biocompatibility and have good potential for biomedical applications.
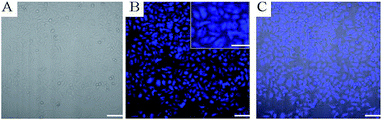
Considering the high water dispersibility, good fluorescence and excellent biocompatibility of the TPEV–PEG copolymer, potential applications in cell imaging together with the cell uptake effect were further investigated by confocal laser scanning microscopy (CLSM) as shown in Fig. 4 after they were taken up by HepG2 cells.34,45,46 The bright dots are the HepG2 cells, and the presence of the obvious blue fluorescence implied that the TPEV–PEG FONs have been taken up by HepG2 cells. With careful observation, the TPEV–PEG FONs were seen to be mainly located at the cytoplasm, and the centre areas of the dots with relatively weak fluorescence intensity should be the cell nuclei (Fig. 4B).11 From the above preliminary results, it was concluded that TPEV–PEG FONs could be easily internalized by cells with most of them locating at the cytoplasm. Considering the size of the TPEV–PEG FONs and the nucleus pore, it was possible for these FONs to be taken up by endocytosis of the cells.47 Combining the merits of AIE and PEG with good fluorescent features, high water dispersibility and excellent biocompatibility, the obtained TPEV–PEG FONs were considered to be biocompatible enough for bioimaging applications. Finally, in consideration of the controllability of RAFT polymerization, other polymerizable AIE dyes with different optical properties and numerous monomers with different functional groups could also be easily incorporated into FON polymers, thus, it was expected that multifunctional imaging and theranostic platforms could be obtained by controllable polymerization of polymerizable AIE dyes and some multifunctional monomers.
4. Conclusions
In summary, we synthesized a hydrophobic TPEV AIE dye with a vinyl end functional group by a “one-step” Suzuki coupling reaction of 4-vinylphenylboronic acid and bromotriphenylethylene with high yield, which subsequently participated in RAFT polymerization with a widely used biomedical molecule (PEGMA) monomer to obtain the TPEV–PEG copolymer with transformed side fluorescent groups. The Mn value of the obtained copolymer was about 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 with a narrow PDI of about 1.30, and the molar fraction of hydrophobic TPEV dye and hydrophilic PEG segment in the copolymer was calculated as about 19.2% and 80.8% on the basis of the 1H NMR spectrum, respectively. The as-prepared amphiphilic TPEV–PEG copolymers tended to self-assemble into stable FONs in aqueous solution, with the hydrophobic AIE core covered by a hydrophilic PEG shell. Moreover, in consideration of the favourable properties of TPEV–PEG FONs with high water dispersibility, bright fluorescence and excellent biocompatibility, they have promising potential for application in the bioimaging field. In view of the controllability of RAFT polymerization, various polymerizable AIE dyes can also be incorporated into the polymer through RAFT polymerization to prepare multifunctional FONs, which is very important for the construction of multifunctional theranostics systems. It was concluded that RAFT polymerization by employing various polymerizable AIE dyes should be a facile and efficient strategy for the fabrication of multifunctional AIE-based FONs with other components, such as targeting agents, drugs, genes or other imaging agents, and these FONs are expected to show great potential for various biomedical applications.
800 g mol−1 with a narrow PDI of about 1.30, and the molar fraction of hydrophobic TPEV dye and hydrophilic PEG segment in the copolymer was calculated as about 19.2% and 80.8% on the basis of the 1H NMR spectrum, respectively. The as-prepared amphiphilic TPEV–PEG copolymers tended to self-assemble into stable FONs in aqueous solution, with the hydrophobic AIE core covered by a hydrophilic PEG shell. Moreover, in consideration of the favourable properties of TPEV–PEG FONs with high water dispersibility, bright fluorescence and excellent biocompatibility, they have promising potential for application in the bioimaging field. In view of the controllability of RAFT polymerization, various polymerizable AIE dyes can also be incorporated into the polymer through RAFT polymerization to prepare multifunctional FONs, which is very important for the construction of multifunctional theranostics systems. It was concluded that RAFT polymerization by employing various polymerizable AIE dyes should be a facile and efficient strategy for the fabrication of multifunctional AIE-based FONs with other components, such as targeting agents, drugs, genes or other imaging agents, and these FONs are expected to show great potential for various biomedical applications.
Acknowledgements
This research was supported by the National Science Foundation of China (No. 21474057, 21104039, 21134004, 51363016), and the National 973 Project (No. 2011CB935700), the Natural Science Foundation of Guangdong Province (S2013010013580).References
- D. Quémener, T. Davis, C. Barner-Kowollik and M. Stenzel, Chem. Commun., 2006, 42, 5051–5053 RSC
.
- M. Kade, D. Burke and C. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS PubMed
.
- H. Li, B. Yu, H. Matsushima, C. Hoyle and A. Lowe, Macromolecules, 2009, 42, 6537–6542 CrossRef CAS
.
- C. Becer, R. Hoogenboom and U. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900–4908 CrossRef CAS PubMed
.
- M. Li, P. De, S. Gondi and B. Sumerlin, Macromol. Rapid Commun., 2008, 29, 1172–1176 CrossRef CAS PubMed
.
- C. Boyer, V. Bulmus, T. Davis, V. Ladmiral, J. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS PubMed
.
- S. Perrier and P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5347–5393 CrossRef CAS PubMed
.
- S. Wang, C. Fu, Y. Zhang, L. Tao, S. Li and Y. Wei, ACS Macro Lett., 2012, 1, 1224–1227 CrossRef CAS
.
- Y. Zhang, Y. Zhao, B. Yang, C. Zhu, Y. Wei and L. Tao, Polym. Chem., 2014, 5, 1857–1862 RSC
.
- X. Shen, Y. Shi, B. Peng, K. Li, J. Xiang, G. Zhang, Z. Liu, Y. Chen and D. Zhang, Macromol. Biosci., 2012, 12, 1583–1590 CrossRef CAS PubMed
.
- X. Zhang, M. Liu, B. Yang, X. Zhang, Z. Chi, S. Liu, J. Xu and Y. Wei, Polym. Chem., 2013, 4, 5060–5064 RSC
.
- X. Zhang, X. Zhang, B. Yang, J. Hui, M. Liu, W. Liu, Y. Chen and Y. Wei, Polym. Chem., 2014, 5, 689–693 RSC
.
- X. Zhang, X. Zhang, B. Yang, J. Hui, M. Liu, Z. Chi, S. Liu, J. Xu and Y. Wei, Polym. Chem., 2014, 5, 683–688 RSC
.
- X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398–4414 RSC
.
- H. Tong, Y. Hong, Y. Dong, Y. Ren, M. Haeussler, J. Lam, K. Wong and B. Tang, J. Phys. Chem. B, 2007, 111, 2000–2007 CrossRef CAS PubMed
.
- H. Tong, Y. Dong, Y. Hong, M. Haeussler, J. Lam, H. Sung, X. Yu, J. Sun, I. Williams, H. Kwok and B. Tang, J. Phys. Chem. C, 2007, 111, 2287–2294 CAS
.
- R. Hu, N. Leung and B. Tang, Chem. Soc. Rev., 2014, 43, 4494–4562 RSC
.
- X. Zhang, Z. Chi, H. Li, B. Xu, X. Li, W. Zhou, S. Liu, Y. Zhang and J. Xu, Chem.–Asian J., 2011, 6, 808–811 CrossRef CAS PubMed
.
- X. Zhang, Z. Chi, H. Li, B. Xu, X. Li, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2011, 21, 1788–1796 RSC
.
- X. Zhang, Z. Chi, B. Xu, C. Chen, X. Zhou, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem., 2012, 22, 18505–18513 RSC
.
- J. Liu, J. Lam and B. Tang, J. Inorg. Organomet. Polym., 2009, 19, 249–285 CrossRef CAS
.
- J. Liu, J. Lam and B. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS PubMed
.
- L. Tang, J. Jin, A. Qin, W. Yuan, Y. Mao, J. Mei, J. Sun and B. Tang, Chem. Commun., 2009, 45, 4974–4976 RSC
.
- R. Hu, J. Maldonado, M. Rodriguez, C. Deng, C. Jim, J. Lam, M. Yuen, G. Ramos-Ortiz and B. Tang, J. Mater. Chem., 2012, 22, 232–240 RSC
.
- R. Hu, J. Lam, Y. Yu, H. Sung, I. Williams, M. Yuen and B. Tang, Polym. Chem., 2013, 4, 95–105 RSC
.
- X. Zhang, M. Liu, B. Yang, X. Zhang and Y. Wei, Colloids Surf., B, 2013, 112, 81–86 CrossRef CAS PubMed
.
- Z. Huang, X. Zhang, X. Zhang, C. Fu, K. Wang, J. Yuan, L. Tao and Y. Wei, Polym. Chem., 2015, 6, 607–612 RSC
.
- L. Tao, J. Liu and T. Davis, Biomacromolecules, 2009, 10, 2847–2851 CrossRef CAS PubMed
.
- Z. Huang, X. Zhang, X. Zhang, S. Wang, B. Yang, K. Wang, J. Yuan, L. Tao and Y. Wei, RSC Adv., 2015, 5, 65884–65889 RSC
.
- Z. Huang, X. Zhang, X. Zhang, B. Yang, Y. Zhang, K. Wang, J. Yuan, L. Tao and Y. Wei, Polym. Chem., 2015, 6, 2133–2138 RSC
.
- D. Jana and B. Ghorai, Tetrahedron Lett., 2012, 53, 196–199 CrossRef CAS PubMed
.
- X. Zhang, Z. Chi, J. Zhang, H. Li, B. Xu, X. Li, S. Liu, Y. Zhang and J. Xu, J. Phys. Chem. B, 2011, 115, 7606–7611 CrossRef CAS PubMed
.
- X. Zhang, X. Zhang, B. Yang, J. Hui, M. Liu, Z. Chi, S. Liu, J. Xu and Y. Wei, Polym. Chem., 2014, 5, 318–322 RSC
.
- X. Zhang, X. Zhang, B. Yang, M. Liu, W. Liu, Y. Chen and Y. Wei, Polym. Chem., 2014, 5, 356–360 RSC
.
- Y. Hong, J. Lam and B. Tang, Chem. Commun., 2009, 45, 4332–4353 RSC
.
- X. Zhang, J. Yin, C. Peng, W. Hu, Z. Zhu, W. Li, C. Fan and Q. Huang, Carbon, 2011, 49, 986–995 CrossRef CAS PubMed
.
- X. Zhang, S. Wang, C. Zhu, M. Liu, Y. Ji, L. Feng, L. Tao and Y. Wei, J. Colloid Interface Sci., 2013, 397, 39–44 CrossRef CAS PubMed
.
- X. Zhang, X. Zhang, S. Wang, M. Liu, L. Tao and Y. Wei, Nanoscale, 2013, 5, 147–150 RSC
.
- X. Zhang, W. Hu, J. Li, L. Tao and Y. Wei, Toxicol. Res., 2012, 1, 62–68 RSC
.
- X. Zhang, H. Qi, S. Wang, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Toxicol. Res., 2012, 1, 201–205 RSC
.
- X. Zhang, J. Hui, B. Yang, Y. Yang, D. Fan, M. Liu, L. Tao and Y. Wei, Polym. Chem., 2013, 4, 4120–4125 RSC
.
- H. Qi, M. Liu, L. Xu, L. Feng, L. Tao, Y. Ji, X. Zhang and Y. Wei, Toxicol. Res., 2013, 2, 427–433 RSC
.
- X. Zhang, S. Wang, M. Liu, J. Hui, B. Yang, L. Tao and Y. Wei, Toxicol. Res., 2013, 2, 335–346 RSC
.
- K. Wang, X. Zhang, X. Zhang, B. Yang, Z. Li, Q. Zhang, Z. Huang and Y. Wei, Macromol. Chem. Phys., 2015, 216, 678–684 CrossRef CAS PubMed
.
- B. Yang, Y. Zhang, X. Zhang, L. Tao, S. Li and Y. Wei, Polym. Chem., 2012, 3, 3235–3238 RSC
.
- X. Zhang, S. Wang, C. Fu, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Polym. Chem., 2012, 3, 2716–2719 RSC
.
- H. Li, X. Zhang, X. Zhang, B. Yang, Y. Yang and Y. Wei, Polym. Chem., 2014, 5, 3758–3762 RSC
.
| This journal is © The Royal Society of Chemistry 2015 |