Graphene-reinforced poly(vinyl alcohol) electrospun fibers as building blocks for high performance nanocomposites
Abstract
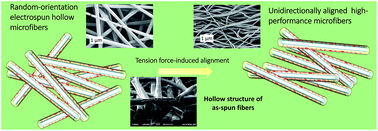
Graphene-containing fibrous structures with a high level of affinity towards a polymer matrix solution have been proved to be promising for high performance macroscopic nanocomposite reinforcement purposes. In this study, for the first time, co-solvent assisted well-dispersed thermally reduced graphene oxide dispersions were successfully prepared. Graphene solutions were then further dissolved in poly(vinyl alcohol) solutions and electrospun, respectively, producing hollow nanofibers, for which key properties, such as solution extensional viscosity and fiber mechanical properties, were studied. The hollowness of the as-spun fibers were comprehensively investigated through a focused ion beam-based advanced cutting and cross-section technique in a dual beam instrument. The effect of reduced graphene oxide content on individual fiber alignment of mats subjected to tension forces was studied via scanning electron microscopy. The analysis revealed that the optimum alignment was achieved at 0.6 wt% graphene-content as-spun mats, in which the Young's modulus was improved by over 60% compared to the neat PVA as-spun mats. The mentioned phenomenon was found as responsible for superior mechanical properties.

 Please wait while we load your content...
Please wait while we load your content...