A simpler and highly efficient protocol for the preparation of biodiesel from soap stock oil using a BBSA catalyst†
Mehulkumar L. Savaliya and
Bharatkumar Z. Dholakiya*
Department of Applied Chemistry, S. V. National Institute of Technology (SVNIT), Ichchhanath, Surat-395007, Gujarat, India. E-mail: bharat281173@gmail.com; msmehulsavaliya@gmail.com; Tel: +91-9428949595
First published on 27th August 2015
Abstract
The present work deals with the synthesis of a highly promising bagasse based solid acid (BBSA) catalyst through sulfonation as well as partial carbonization of bagasse powder and is applied to the esterification of soap stock oil for the production of biodiesel. The highest (%) FFA conversion was observed up to 97.2%. The bagasse based solid acid catalyst revealed promising catalytic activity for esterification of soap stock oil with a 5% catalyst dose (w/w). The BBSA catalyst was duly characterized by FT-IR, XRD, BET, TGA and SEM analysis. Meanwhile, the synthesized biodiesel was well characterized by FT-IR as well as 1H and 13C NMR spectroscopic techniques. Moreover, the fuel properties of the synthesized biodiesel have been measured and compared with ASTM fuel standards.
1. Introduction
Biodiesel is a biomass-based fuel that is considered to be one of the most promising petroleum diesel fuel substitutes.1 Biodiesel offers many merits as an alternative, renewable, nontoxic, biodegradable and eco-friendly fuel.2 Recently, biodiesel has received increased attention because of day by day increases in crude oil prices, limited reservoirs of fossil oil and global environmental issues. There has been a renewed focus on vegetable oils and animal fats to produce biodiesel fuels.3 Biodiesel production from abundant bio-resources has drawn considerable attention from academics as well as the industrial community recently.4 Currently, the global research in the biodiesel industries have been focused on how to convert lower cost feedstocks especially renewable wastes and non-traditional lipids into biodiesel products via simplified technology i.e. cheaply and easily available catalyst and reactants, economically viable and environmentally friendly, etc.5–7 Currently, biodiesel is being obtained mainly from high quality, refined vegetable oils such as rapeseed, sunflower,8 soybean and palm oil. However, the potential of those raw materials is limited due to food vs. fuel issue, the limited crop land, yield and the relative high market price, which lowers biodiesel profit margin to the manufacturers. An alternative feedstock for sustainable first generation biofuels is the exploitation of waste oils and fats such as waste frying oil,9 and animal fat10 or the use of non-edible oil crops such as jatropha.11,12In the commercial production of biodiesel most catalysts preferred for transesterification reactions are homogeneous alkali catalysts. Sodium hydroxide, sodium methoxide, potassium hydroxide, and potassium methoxide are the usually used homogeneous alkali catalysts.13 Though, homogeneous alkali catalysts offer numerous advantages including high catalytic efficiency and fast reaction rate. However, homogeneous catalysts are not recyclable and complicate the downstream process of final products.14
At present, the main drawback for utilization of biodiesel is its higher cost than petroleum derived diesel.15,16 The higher cost of biodiesel is mainly due to its being produced from high quality virgin oil with lower levels of free fatty acid contents. A way for reducing biodiesel cost is to utilize lower cost feedstock comprising higher levels of FFAs, recycled or waste oil and byproducts of vegetable oils refineries.17,18 Physical and chemical properties of any feedstock that influence the potential biodiesel production are free fatty acid (FFA) content, moisture content and other impurities, titer and calorific content.13 The physico-chemical properties of acid soap stock (edible oil refinery waste) oil is illustrated in Table 1.19
| Entry | Properties | Values (%) |
|---|---|---|
| 1 | Free fatty acid | 35.7 mg KOH g−1 |
| 2 | Acid value | 265.5 mg KOH g−1 |
| 3 | Iodine value | 194.7 g I2/100 g |
| 4 | Saponification value | 289 mg KOH g−1 |
| 5 | Peroxide value | 59.25 meq. kg−1 |
| 6 | Refractive index | 0.464 |
| 7 | Specific gravity | 0.92 |
| 8 | Colour | Dark reddish brown colour |
| 9 | pH | 4.82 |
| 10 | Taste | Mild taste |
| 11 | Viscosity | 74 |
Solid acid catalysts render significant advantages of eliminating separation, corrosion, toxicity and environmental problems. Therefore, they have recently attracted considerable attention of biodiesel manufacturers. A few reports have given importance of solid acid catalyst for biodiesel synthesis. Apart from recyclability and reusability, an ideal solid acid catalyst for biodiesel preparation should offers high stability, numerous strong acid sites, large pore volumes, hydrophobic surface and economically viable.20
In concern of these studies, bagasse based solid acid catalyst has been prepared from bagasse powder using very conventional synthetic approach and applied to the esterification of soap stock oil regarding biodiesel synthesis. Bagasse based solid acid catalyst offers very good efficiency to catalyzed esterification of high FFA containing waste oils. In addition, being a heterogeneous catalyst, BBSA catalyst can be recovered and reused several time after simple recovery and regeneration. Generally the performance of acid catalyst doesn't influenced by presence of FFAs in feedstocks. Because it can simultaneously convert free fatty acids and triglycerides to biodiesel by esterification and transesterification respectively.
2. Materials and methods
2.1. General
FT-IR spectra were recorded on a (Model RZX Perkin Elmer) FT-IR spectrophotometer. Powder X-ray diffraction (XRD, Bruker D8 ADVANCE and PW 1830) pattern was obtained by using Cu Kα (λ = 1.54056 Å) radiation. Specific surface area (SBET) was calculated from the linear part by BET equation. Total acidity of BBSA catalyst was determined through neutralization titration. The structural and surface morphology of the catalysts were characterized by scanning electron microscope (SEM; Hitachi S3400 FEG). Thermal stability of catalyst was determined by thermo gravimetric analysis (Model: Perkin Elmer TGA-7). 1H and 13C NMR of oil and biodiesel samples were recorded on (Model: RZX BRUKER ADVANCE) 400 MHz FT-NMR spectrometer using TMS as an internal standard. The composition of soap stock biodiesel was determined by using well established GC analysis (YL 65000GC). Reaction monitoring was accomplished by TLC monitoring on SILG/UV 254 plates.2.2. Materials
Soap stock oil was obtained as a munificence gift from Shree Khodal Oil Refinery Ltd, Gondal, Gujarat, India. Methanol (HPLC grade) was purchased from Sigma Aldrich. Raw bagasse material was collected from Shri Ukai Pradesh Sahakari Khand Udyog Mandli Ltd, Kamrej, Surat, Gujarat. Sulphuric acid (synthesis grade) was purchased from Aashka Scientific Co Ltd, Surat, Gujarat, India.2.3. Preparation of BBSA catalyst
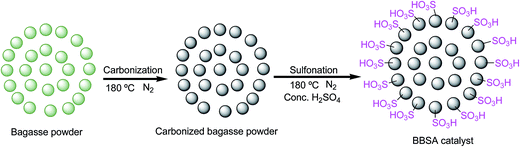
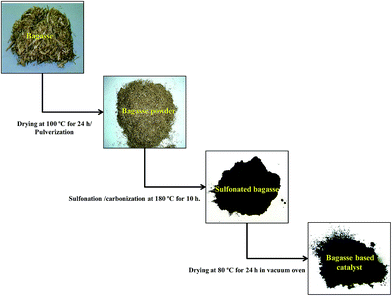
A 500 ml flat bottom flask was charged with concentrated sulfuric acid (30 ml). Bagasse powder (5 g) was added and heated at 180 °C under N2 flow to produce a black solid (in situ partial carbonization and sulfonation) for 10 h. Then the mixture was cooled to room temperature and diluted with distilled water (500 ml) to produce a black precipitate. The precipitate was collected by vacuum filtration and washed repeatedly with hot distilled water until impurities like sulfate ions were no longer detected in the filtrate. The resulting black solid was dried at 80 °C for 24 h in vacuum oven to remove water absorbed on the catalyst. The total yield of the catalyst was 25–35 wt% based on the weight of the raw material. General scheme for the synthesis of bagasse based solid acid catalyst from bagasse powder is given in Fig. 1.Whereas, the schematic layout for the preparation of BBSA catalyst is represented in Fig. 2.
2.4. BBSA catalyzed esterification of soap stock oil to biodiesel
To understand the influence of oil to methanol molar ratio, reaction temperature, catalyst amount, water and free fatty acids on the yield of acid-catalyzed methyl ester, a laboratory scale reactor consisting of a 500 ml flat bottom flask was used. The free fatty acid esterification process was usually carried out under continuous water removal.19 Water removal during esterification is needed as it is formed as a side product and will reduce the activity of a solid acid catalyst. It can also promote reverse reaction. Besides, esterification is an equilibrium limited reaction in which full conversion can only be achieved when one of the products is removed. To resolve this problem, anhydrous sodium sulphate (0.1 mol) was added in the reaction to trap water formation during reaction. Therefore, in the present study, in a two neck flat bottom flask (equipped with reflux condenser, temperature indicator and continuous water removing system), a mixture of soap stock oil, methanol and bagasse based solid acid catalyst was added and stirred at 65 °C for the appropriate time. Finally, the reaction mixture was cooled to ambient temperature; the bagasse based solid acid catalyst was filtered off. The filtrate was poured in a cold water to get soap stock biodiesel at upper layer. Soap stock biodiesel production has been studied using different soap stock oil to methanol molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 1
6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as well as varying length of reactions time (8, 9, 10, 11, 12 and 13 h). The reaction setup for esterification of soap stock oil is illustrated in Fig. S1.† The (%) FFA conversion was measured by eqn (i).
20) as well as varying length of reactions time (8, 9, 10, 11, 12 and 13 h). The reaction setup for esterification of soap stock oil is illustrated in Fig. S1.† The (%) FFA conversion was measured by eqn (i).
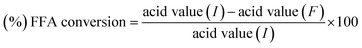 | (i) |
General reaction scheme for esterification of soap stock oil to biodiesel is demonstrated in Fig. 3. Whereas, Fig. S2† shows the soap stock oil and soap stock biodiesel.
2.5. Determination of FAME compositions in soap stock biodiesel
Fatty acid methyl ester compositions was estimated by gas chromatography analysis. The composition of soap stock biodiesel was determined by using well established GC analysis. Sigma Aldrich C8–C24 component FAME mix was used as a standard for identification and quantification of the peaks obtained in the soap stock biodiesel sample on YL 65000GC having Agilent DB-Wax-123-7032 column (30 m × 0.320 mm × 0.25 μm) and flame ionization detector. A sample of 0.6 μl (0.5 mg soap stock biodiesel in 1 ml of hexane) was injected under the split mode of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and injector temperature was maintained at 260 °C using nitrogen as a carrier gas. The oven was kept at 140 °C for 5 min and then heated up to 240 °C at the rate of 4 °C min−1 with the holding time of 20 min. The detector temperature was kept to 270 °C. The fatty acid methyl ester peaks in the soap stock biodiesel samples were identified and compared with the peaks obtained in the GC chromatogram of the internal standard. The representative gas chromatogram of soap stock biodiesel is demonstrated in Fig. S3.†
1 and injector temperature was maintained at 260 °C using nitrogen as a carrier gas. The oven was kept at 140 °C for 5 min and then heated up to 240 °C at the rate of 4 °C min−1 with the holding time of 20 min. The detector temperature was kept to 270 °C. The fatty acid methyl ester peaks in the soap stock biodiesel samples were identified and compared with the peaks obtained in the GC chromatogram of the internal standard. The representative gas chromatogram of soap stock biodiesel is demonstrated in Fig. S3.†
From the GC-FID chromatogram of soap stock biodiesel, it has been found that the synthesized soap stock biodiesel contains different kind of fatty acid methyl ester (FAMEs) comprising methyl oleate, methyl palmitate, methyl linoleate, methyl linolenate and methyl behenate.
3. Result and discussion
Generally, the esterification reaction involves several critical parameters which significantly influence the final conversion and yield.13 Piyanuch et al. have conducted the esterification of high free fatty acid coconut oil for biodiesel synthesis using sulphuric acid catalyst.21 Nakpong et al. have carried out esterification reaction using different catalyst concentrations (0.5, 0.6, 0.7 and 0.8% H2SO4) and reaction times (30, 60, 90 and 120 min) for investigating their effect on the reduction of the acid value of oil. The methanol to oil molar ratio and reaction temperature were 0.35 v/v and 60 °C respectively. They found that ester formation rate increased with increasing catalyst concentration.7Wen et al. have explained more precisely, various carbohydrate-derived catalysts, especially starch derived catalyst, were shown to be highly effective in converting high FFA-containing waste oils to biodiesel by simultaneous esterification and transesterification. Under the optimized reaction conditions, usage of the most effective starch-derived catalyst for biodiesel production from waste cooking oils containing 27.8 wt% FFAs afforded the methyl ester yield of about 92% after 8 h. This catalyst also manifested very excellent operational stability.20 The result of FFA conversion (%) with varying soap stock oil to methanol molar ratio and reaction time (h) have been summarized in Table 2.
| Entry | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M molar ratio M molar ratio |
Catalyst loading (% w/w) | Reaction temp. (°C) | Reaction time (h) | FFA conversion (%) |
|---|---|---|---|---|---|
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
5 | 65 | 8 | 58.4 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
5 | 65 | 8 | 65.7 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
5 | 65 | 8 | 74.3 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
5 | 65 | 8 | 80.2 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
5 | 65 | 8 | 90.2 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
5 | 65 | 8 | 91.6 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
5 | 65 | 8 | 93.2 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
5 | 65 | 8 | 94.0 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
5 | 65 | 8 | 94.6 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 8 | 95.3 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
5 | 65 | 8 | 95.2 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
5 | 65 | 8 | 94.9 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
5 | 65 | 8 | 94.5 |
| 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
5 | 65 | 8 | 94.2 |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
5 | 65 | 8 | 94.1 |
| 16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 9 | 96.5 |
| 17 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 10 | 96.9 |
| 18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 11 | 97.2 |
| 19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 12 | 97.2 |
| 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
5 | 65 | 13 | 97.2 |
From Table 2, it has been found that as methanol to oil molar ratio increases, it directly affect the % FFA conversion. The (%) FFA conversion is increases with increasing in methanol to oil molar ratio. The highest (%) FFA conversion was found with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol molar ratio. The soap stock oil to methanol molar ratio below 1
15 soap stock oil to methanol molar ratio. The soap stock oil to methanol molar ratio below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 directly facilitates the reversible reaction. Therefore, the main reason behind decrement in the % FFA conversion below 1
15 directly facilitates the reversible reaction. Therefore, the main reason behind decrement in the % FFA conversion below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol molar ratio is the reversible nature of the esterification reaction. It has been also found that the decrement in the % FFA conversion beyond the 1
15 soap stock oil to methanol molar ratio is the reversible nature of the esterification reaction. It has been also found that the decrement in the % FFA conversion beyond the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol molar ratio. This may be attributed to the polar nature of catalyst and methanol. Therefore, as the methanol to oil molar ratio increases, the polarity of the reaction goes on increases. The mechanism of esterification reaction suggests that the protonation of soap stock oil should be first and foremost. However, the polarity of the esterification goes on increases as the concentration of methanol increases. Therefore, instead of soap stock oil phase, the catalyst directly turns towards the methanol phase. Therefore, the decrement in the (%) FFA conversion was observed beyond the 1
15 soap stock oil to methanol molar ratio. This may be attributed to the polar nature of catalyst and methanol. Therefore, as the methanol to oil molar ratio increases, the polarity of the reaction goes on increases. The mechanism of esterification reaction suggests that the protonation of soap stock oil should be first and foremost. However, the polarity of the esterification goes on increases as the concentration of methanol increases. Therefore, instead of soap stock oil phase, the catalyst directly turns towards the methanol phase. Therefore, the decrement in the (%) FFA conversion was observed beyond the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol molar ratio.
15 soap stock oil to methanol molar ratio.
Moreover, the esterification of soap stock oil was also studied using different reaction time comprising 8, 9, 10, 11, 12 and 13 h. From, the experimental results, it has been found that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 oil to methanol molar ratio shows maximum FFA conversion (95.3%). Therefore, 1
15 oil to methanol molar ratio shows maximum FFA conversion (95.3%). Therefore, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 oil to methanol molar ratio was selected as an optimum ratio to study biodiesel synthesis using different reaction time. The esterification of soap stock oil to biodiesel has been also studied by different length of reaction times (8, 9, 10, 11, 12 and 13 h) in order to study the influence of reaction time on the FFA (%) conversion. From the experimental result, it can be concluded that, in case of 9 h reaction time, the highest FFA conversion (%) observed was 96.5%. In case of 10 h reaction time, the highest FFA conversion (%) observed was 96.9%. Whereas, in case of 11 h reaction time, the highest FFA conversion (%) observed was 97.2%. However, the reaction time beyond 11 h doesn't show any remarkable effect on the FFA conversion (%). The similar (%) FFA conversions were obtained using 12 h and 13 h reaction time at identical reaction conditions. It can be clearly evidenced from experimental results, the best condition to develop efficient process for the preparation of soap stock biodiesel from high FFA containing soap stock oil are, 1
15 oil to methanol molar ratio was selected as an optimum ratio to study biodiesel synthesis using different reaction time. The esterification of soap stock oil to biodiesel has been also studied by different length of reaction times (8, 9, 10, 11, 12 and 13 h) in order to study the influence of reaction time on the FFA (%) conversion. From the experimental result, it can be concluded that, in case of 9 h reaction time, the highest FFA conversion (%) observed was 96.5%. In case of 10 h reaction time, the highest FFA conversion (%) observed was 96.9%. Whereas, in case of 11 h reaction time, the highest FFA conversion (%) observed was 97.2%. However, the reaction time beyond 11 h doesn't show any remarkable effect on the FFA conversion (%). The similar (%) FFA conversions were obtained using 12 h and 13 h reaction time at identical reaction conditions. It can be clearly evidenced from experimental results, the best condition to develop efficient process for the preparation of soap stock biodiesel from high FFA containing soap stock oil are, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol molar ratio, 65 °C reaction temperature, 11 h reaction time and 5% (w/w) of catalytic material.
15 soap stock oil to methanol molar ratio, 65 °C reaction temperature, 11 h reaction time and 5% (w/w) of catalytic material.
3.1. Chemistry involved in esterification soap stock oil to biodiesel
The mechanism of esterification of free fatty acid and methanol could be accomplished via three steps, in first step acid catalyst protonate the free fatty acid molecule via liberation of the proton, the protonated acid molecule will remove one molecule of water, followed by nucleophilic attack of oxygen will take place, which is lying in the hydroxyl group of methanol to form tetrahedral intermediate. Finally, deprotonation of a tetrahedral intermediate leads to generate corresponding methyl ester (soap stock biodiesel). The general reaction mechanism scheme has been demonstrated in Fig. S4.†3.2. Confirmations of active functional groups on BBSA surface
FT-IR spectroscopy is an important tool in the hand of chemist to measure distinguished functional groups present in the synthesized species. From the FT-IR spectrum of catalyst, it has been found that, BBSA catalyst reveals different bands indicating present of SO3H groups on the catalyst surface. FT-IR spectrum of BBSA is confirmed by the presence of characteristics bands at 1284.63 cm−1 (C–O–H bending), 1179.51 cm−1 (SO2 asymmetric stretching provided by presence of SO3H group), 1010.73 cm−1 (SO2 symmetrical stretching), 887.28 cm−1 (C–O–C stretching), 852.56 cm−1 (C–C stretching) and 663.53, 614.35, 578.66, 501.51, 457.14 cm−1 (skeleton modes of pyranol ring), respectively.22 FT-IR spectrum of BBSA catalyst is given in Fig. S5.†3.3. Powder X-ray diffraction study of BBSA catalyst
X-ray powder diffraction (XRD) is a rapid analytical technique primarily used for phase identification of a crystalline material. The texture properties of BBSA catalyst were examined by XRD analysis and their pattern is demonstrated in Fig. S6.† The spectrum exhibited typical diffraction peaks indicating amorphous nature of BBSA catalyst. The broad diffraction peak (2θ = 15–30°) can be attributed to the amorphous carbon structures. The weak and broad diffraction peak (2θ = 40–50°) is due to the axis of the carbon composed of aromatic carbon sheets oriented in a considerably random fashion.233.4. Morphological study of BBSA catalyst
The application of scanning electron microscopy has been proven very fast and convenient to determine the surface structure of synthesized catalyst. The morphology and surface structure of fresh and spent bagasse based solid acid catalysts were predicted from the SEM micrographs, it has been found that synthesized catalyst particles exhibited an irregular flakes like framework structure after carbonization and sulfonation. Moreover, it could be found that the particles size became smaller and particles tended to aggregate after the sulfonation with concentrated sulfuric acid. However, in case of morphology of spent catalyst, it has been found that the morphology and irregular flakes like framework structure is slightly disturbed during the reaction. No major significant changes were observed in SEM micrographs of fresh and spent catalysts, they almost revealed retention of framework structure throughout the reactions. Shu et al. have explained the morphology of carbon based solid acid catalyst using D-glucose as the carbon precursor. The obtained D-glucose catalyst showed an irregular compact structure which looked like a grain with a size larger than 1 μm, and no obvious pores can be seen. The different structures in the asphalt and D-glucose catalyst could be explained as follows. The main components in these two carbon sources were different, and it was probable that straight chain aliphatic hydrocarbon polymers can more easily form pores than ring hydrocarbon polymers when they were incomplete carbonized. The larger amounts of pores and larger pore size would increase the accessibility of sulfuric acid into the carbon powder bulk, which would give a higher concentration of covalently bonded carbon with –SO3H group.24 SEM micrographs of fresh and spent bagasse based solid acid catalysts are demonstrated in Fig. 4.3.5. Surface area determination of BBSA catalyst
BET analysis provides precise specific surface area evaluation of materials by nitrogen multilayer adsorption measured as a function of relative pressure using a fully automated analyzer. The technique encompasses external area and pore area evaluations to determine the total specific surface area in m2 g−1 yielding important information in studying the effects of surface porosity and particle size in variety of applications. BET surface area (SBET) of BBSA catalyst is found to be 1.268 m2 g−1. N2 adsorption–desorption isotherm of bagasse based solid acid catalyst was found typically of type-VI at lower p/po values suggesting the presence of nonporous phase in BBSA catalyst. The lower pore volume (0.0033 cm3 g−1) and surface area indicates the presence of SO3H groups on the pore surface of bagasse molecules. This fact is in accordance with the reported literature.25 N2 adsorption–desorption isotherm of BBSA catalyst has been demonstrated in Fig. S7.†3.6. Determination of thermal stability of BBSA
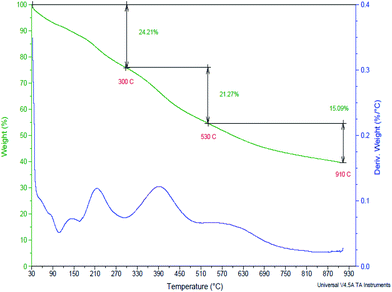
Thermogravimetric analysis (TGA) is an analytical technique used to determine a material's thermal stability and its fraction of volatile components by monitoring the weight change that occurs as a specimen is heated. The thermal stability of bagasse based solid acid catalyst was determined by thermo gravimetric analysis. From the TGA thermogram of bagasse based solid acid catalyst, it has been found that the 24.2% weight loss occurs in a temperature range of 30 to 300 °C is attributed to the lignin decomposition, 21.2% weight loss occurs in a temperature range of 300 to 530 °C is attributed to the cellulose decomposition and 15.0% weight loss occurs in a temperature range of 530 to 910 °C is attributed to the hemicelluloses decomposition. In present case, all esterification reactions are operated at 65 °C. Whereas, the weight loss (24.2%) has been seen in the range of temperature 30 to 300 °C. From the TGA thermogram, it has been found that the thermal stability of catalyst is not much affected at 65 °C reaction temperature. Therefore, the bagasse based solid acid catalyst offers a quite better thermal stability also. Liu et al. have prepared an activated carbon (AC) based solid acid catalyst. The weight loss process of AC–SO3H below 150 °C was similar to that of pure AC. However, the thermogravimetric curve of AC–SO3H at higher temperatures showed a more significant weight loss compared to that of AC, which is mainly due to the gradual desorption and thermal decomposition process of PhSO3H group.26 The TGA thermogram of bagasse based solid acid catalyst is demonstrated in Fig. 5.3.7. Determination of acidity in BBSA catalyst
In order to conduct acid catalyzed synthetic organic transformations, the measurement of total acidity of the catalyst is quite important. The total acidity of the bagasse based solid acid catalyst was 1.9 mmol g−1, which was determined through the neutralization titration. The titration was carried out as follows: catalytic material (40 mg) and 2 N aqueous NaCl (4 ml) were stirred at room temperature for 24 h. The solids were filtered off and washed twice with distilled water (5 ml). The combined filtrate was titrated with 0.01 N NaOH using phenol red as an indicator.27 In order to measure the sulfonic acid density, the sulfur content of BBSA catalyst has been measured by elemental analysis. The bagasse based solid acid catalyst shows that the sulfur content is about 2.9 wt%. Since all sulfur atoms in BBSA catalyst are found as a –SO3H groups. Based on the sulfur content, the mass content of –SO3H groups as well as the –SO3H acid density is found to be about 7.33% and 0.90 mmol g−1 respectively. It has been clearly evidenced from the titration that a total acidity of BBSA catalyst is about 1.9 mmol g−1, which is much higher than that calculated from the sulfur content. Dong et al. have well explained that due to a large fraction of the strong acid groups are likely associated with weak acid groups such as phenolic and carboxylic groups, which imparts polarity to the carbonaceous catalytic material. However, only the –SO3H acid groups are active for the esterification reaction. In addition, such weak acid groups are useful to improve the catalytic activity by increasing the weak acid sites as well as to facilitate the adsorption of reactants on the surface of catalytic material.283.8. FT-IR analysis of soap stock oil
FTIR spectrum of soap stock oil is confirmed with the presence of 2924.09 cm−1 (CH3 stretching), 2854.65 cm−1 (CH2 stretching), 2360.87 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1705.07 cm−1 (C
C stretching), 1705.07 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1458.18 cm−1 (CH bending), 1180.44 cm−1 (C–O) and 717.52 cm−1 (CH rocking) respectively.22 FT-IR spectrum of soap stock oil is given in Fig. S8.†
O), 1458.18 cm−1 (CH bending), 1180.44 cm−1 (C–O) and 717.52 cm−1 (CH rocking) respectively.22 FT-IR spectrum of soap stock oil is given in Fig. S8.†
3.9. FT-IR analysis of soap stock biodiesel
FTIR spectrum of soap stock biodiesel is confirmed with the presence of 2931.80 cm−1 (CH3 stretching), 2854.65 cm−1 (CH2 stretching), 1735.93 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1442.75 cm−1 (CH bending), 1242.16 cm−1 (C–O) and 756.10 cm−1 (CH rocking) respectively.22 FT-IR spectrum of soap stock biodiesel is given in Fig. S9.†
O), 1442.75 cm−1 (CH bending), 1242.16 cm−1 (C–O) and 756.10 cm−1 (CH rocking) respectively.22 FT-IR spectrum of soap stock biodiesel is given in Fig. S9.†
3.10. 1H NMR characterization of soap stock oil
The application of 1H NMR analysis is very handy and quick in monitoring a reaction progress, because a trace sample can be extracted from the batch reaction at any given time and the 1H NMR analysis provides quantitative information regarding the chemical species present in the reaction. The biodiesel sample was solubilized in a DMSO solvent prior to analysis. The purity of soap stock oil is confirmed by the presence of characteristics peaks corresponding to 5.26–5.35 (CH2–CO–O– protons) ppm, 3.56–3.57 (O–CH2) ppm, 2.72–2.75 (α-CH2 protons) ppm, 2.25–2.51 (β-CH2 protons) ppm and 1.95–2.19 (CH3 protons) ppm.29 1H NMR spectrum of soap stock oil is given in Fig. S10.†3.11. 1H NMR analysis of synthesized soap stock biodiesel
Proton NMR analysis provides a good probe for biodiesel since 1H is the most naturally abundant and most sensitive NMR active isotope. Relatively narrow line widths of a few Hertz are obtained for 1H spectra so that magnetically unique nuclei are resolved at many field strengths. The biodiesel sample was solubilized in a CDCl3 solvent prior to analysis. The purity of soap stock biodiesel is further confirmed by the presence of characteristics peaks at 3.57 (CH3O–methoxy protons) ppm, 3.32 (α-CH2 protons) ppm and 4.36 (unsaturated olefinic –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– protons) ppm.29 1H NMR spectrum of synthesized soap stock biodiesel has been given in Fig. S11.†
CH– protons) ppm.29 1H NMR spectrum of synthesized soap stock biodiesel has been given in Fig. S11.†
3.12. 13C NMR characterization soap stock oil
The purity of soap stock oil is further confirmed by the presence of characteristics peaks corresponding to 172.39–174.32 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 127.64–129.58 ppm (olefinic carbons), 69.26 (CH–O carbons), 62.62–65.42 (CH2O carbon), 54.79 ppm (O–CH3 carbon), 51.00 ppm (CH3 carbons), 38.82–40.08 (DMSO solvent) and 28.88–33.60 ppm (aliphatic carbons) respectively.30 13C NMR spectrum of synthesized soap stock biodiesel is given in Fig. S12.†
O), 127.64–129.58 ppm (olefinic carbons), 69.26 (CH–O carbons), 62.62–65.42 (CH2O carbon), 54.79 ppm (O–CH3 carbon), 51.00 ppm (CH3 carbons), 38.82–40.08 (DMSO solvent) and 28.88–33.60 ppm (aliphatic carbons) respectively.30 13C NMR spectrum of synthesized soap stock biodiesel is given in Fig. S12.†
3.13. 13C NMR analysis of synthesized soap stock biodiesel
The progress of esterification of soap stock oil was also determined by 13C NMR spectroscopy. Purity of soap stock biodiesel is confirmed by the presence of characteristics peaks, including, 127.78 ppm (olefinic carbons), 76.81–77.45 (CDCl3 solvent), 54.79 ppm (O–CH3 carbon), 49.74–51.43 ppm (CH3 carbons) and 22.42–34.00 ppm (aliphatic carbons) respectively.30 13C NMR spectrum of synthesized soap stock biodiesel is given in Fig. S13.†3.14. Repeatability study of BBSA catalyst
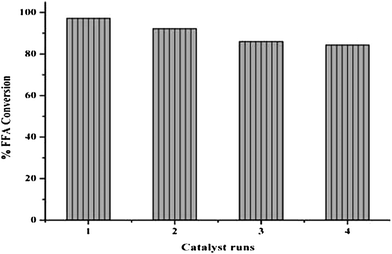
In order to control the cost of biodiesel production, catalyst reusability is quite important. Therefore, in this segment, bagasse based solid acid catalyst was filtered off from the reaction media and washed at twice with methylene dichloride solvent for removal of impurity traces such as surface bound moisture, unreacted free fatty acids, gums, phospholipids and methanol. Finally washed catalyst was place in a tray dryer at 80 °C for 10 h to allow removal of solvent and activation of acid sites on bagasse surface. Results clearly indicate that catalyst can be reused for three times without significant loss of activity. However, the little decrement in the biodiesel yield has been observed in subsequent runs. The observed catalyst deactivation could be due to modification of catalyst structure or leaching of active sites at given reaction temperature. It has been found that bagasse exhibited its structure through the reaction without any change and also it is clearly evidenced from spectral characterization of catalyst (Fig. 4).Fresh bagasse based solid acid catalyst can give maximum % FFA conversion up to 97.2%. While, it's first, second and third cycle can give maximum % FFA conversions up to 92.2%, 86.0% and 84.3% respectively.
Savaliya et al. have synthesized silica based boron trisulfonic acid catalyst and tested it for biodiesel synthesis using refined soybean oil. They studied the reusability of the catalyst in transesterification of refined soybean oil. From the experiments, they concluded that the catalyst can be reuse three times without significant loss in their activity.19 BBSA catalyst reusability study is represented in Fig. 6.
3.15. Comparison of catalytic activity of BBSA with other reported solid acid catalyst
The comparison of catalytic activity of BBSA catalyst with other reported identical class of catalyst is illustrated in Table 3.| Sr. no. | Type of catalyst | Reaction conditions | Biodiesel yield (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Catalyst% (w/w) | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M ratio (mol) M ratio (mol) |
Time (h) | ||||
| 1 | BBSA | 65 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
11 | 97.2 | Present case |
| 2 | EBD 100 | 65 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
24 | 100 | 31 |
| 3 | Sulfonated glucose–starch | 80 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
12 | 90 | 32 |
| 4 | Waste carbon based catalyst | 110 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | 84 | 33 |
| 5 | Waste palm tree biomass based catalyst | 100 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
5 | 98.5 | 34 |
| 6 | Activated carbon fiber supported HPAs | 60 | 1.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 97 |
6 | 76 | 35 |
However, other carbon-based solid acid catalysts prepared with the identical method using sucrose, glucose,36–40 corn straw,41 starch, cellulose38 and hardwood char materials42 and applied for the esterification of free fatty acids with methyl alcohol or other alcohol. The catalytic activity tests showed that the conversion of acids was 80–90%.43 In fact that the reactants and reaction conditions were quite different from each other; it is quite difficult to conclude which is more better catalyst. However, in our experiments, the (%) FFA conversion is reached about 97.2%, therefore, the bagasse is an optimistic raw material for the preparation of similar carbon-based solid acids and can be used in other acid-catalyzed synthetic organic transformations to replace conventional homogeneous acid catalysts.
From Table 3, it has been found that synthesized catalyst shows very good activity in esterification of soap stock oil for biodiesel production. In present case, the reaction operated at 65 °C temperature, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 oil to methanol molar ratio, 5% (w/w) catalyst and 11 h reaction time using soap stock oil as a feed stock. The highest FFA conversion (%) obtained was 97.2%, which is comparable to other reported results for similar class of acidic catalysts, where relatively harsh reaction condition (high reaction temperature) are employed.32–34
15 oil to methanol molar ratio, 5% (w/w) catalyst and 11 h reaction time using soap stock oil as a feed stock. The highest FFA conversion (%) obtained was 97.2%, which is comparable to other reported results for similar class of acidic catalysts, where relatively harsh reaction condition (high reaction temperature) are employed.32–34
3.16. Determination of fuel properties of soap stock biodiesel
The fuel properties of soap stock biodiesel and its comparison with ASTM standards is summarized in Table 4.| Entry | Properties | Soap stock biodiesel | Test limits | ASTM test methods |
|---|---|---|---|---|
| 1 | Density (40 °C) kg m−3 | 886.2 | 860–900 | D1298 |
| 2 | Flash point (°C) | 180 | >130 | D93 |
| 3 | Kinematic viscosity at 40 °C (mm2 s−1) | 3.9 | 4.5 | D445 |
| 4 | Viscosity, 40 °C (mm2 s−1) | 5.9 | 2.5–6.0 | D445 |
| 5 | Cetane index | 51.2 | 52.0 | D613 |
| 6 | Cloud point (°C) | −4.8 | −15 to 5 | D2500 |
Density, viscosity, flash point, kinematic viscosity, cetane number, cloud and pour point are the most important fuel properties considered in the application of non-edible biodiesels in diesel engines. Many researchers have reported that fuel properties of non-edible biodiesels strongly depends on their fatty acid and chemical composition.44–46 Therefore, before using non-edible-based biodiesels in diesel engines, measuring the fuel properties of selected biodiesels is necessary. The fuel properties of biodiesels are specified by different standardization organizations; the ASTM D6751 and EN14214 are the most popular standards for biodiesel. Viscosity is the most important property of biodiesel since it affects the operation of fuel injection equipment, particularly at low temperature while increasing viscosity affects the fluidity of the fuel. The flash point is a parameter which is considered in the handling, storage and safety of fuels.47
Density of soap stock biodiesel was measured by hydrometer method (D1298). Flash point was measured by Pensky–Martens Closed Cup Tester (D93). Kinematic viscosity (D445) and viscosity (D445) were measured by Aditya 01 Viscometer Bath. Cetane number (D613) was measured by AFIDA 2805 cetane number analyzer. Cloud point (D2500) was measured by Stanhope-seta cloud point analyzer. From Table 4, it has been found that all fuel properties are in accordance with the test limits which are defined by American Society for Testing and Materials (ASTM).
3.17. Economical viability of the adopted protocol
The feedstock contributes to the majority cost of biodiesel synthesis which is already reported to be up to 80% of total cost of biodiesel.48,49 Present investigation demonstrates the utilization of soap stock oil as a feedstock that has the sufficient potential to significantly overcome the production cost of biodiesel. Day by day heterogeneous catalysts are gaining more importance owing to the advantages in terms of down streaming of final products and reusability over the conventionally used homogeneous catalysts. Catalyst from renewable resources comprising biomass has been introduced to offer fully ‘Eco-friendly’ process.50 The soap stock oil is the byproduct of vegetable oil refineries. Therefore, it can be available in the market at very lower cost. As BBSA catalyst has been prepared from bagasse waste, which is waste material of sugar industries. Currently, majority of sugar industries are facing a problem of disposal of bagasse waste, as it is degrades microbially in the monsoon season and produces bad odour, flies, abrasiveness and other toxic chemicals. The pretreatment of bagasse waste using drying and pulverizing will not add significantly cost to the biodiesel synthesis. The preparation of the catalyst by sulfonation and incomplete carbonization would contribute to the cost of synthesis of biodiesel. Moreover, as catalyst being heterogeneous in nature and it could be recovered as well as reuse several times after simpler regeneration as well as reactivation. In short this avenue may prove as a valedictory process for the preparation of lower cost biodiesel.4. Conclusion
In the present investigation, bagasse based solid acid catalyst was successfully synthesized via simpler synthetic approach comprising in situ sulfonation as well as incomplete carbonization at 180 °C temperature and ambient pressure. During this investigation it has been found that the reaction is easy and clean. The total yield of the catalyst was 25–35 wt% based on the weight of the raw material. Furthermore, bagasse based solid acid catalyzed esterification of soap stock oil was investigated. It has been clearly evidenced from experimental result, the optimum reaction condition within the selected parameters ranges were found to be, 65 °C reaction temperature, 11 h reaction time, 5% (w/w) catalytic material loading and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 soap stock oil to methanol ratio, obtaining maximum FFA conversion (97.2%). Synthesized catalyst was also investigated for its reusability study. It has been found that catalyst can be used for three repetitive cycles without significant loss in their activity. There is also reducing cost for downstream processes like separation and recovery of unreacted alcohol that appears on the final cost of biodiesel. In short, a cost effective biodiesel can be produced by using a BBSA catalyzed process from soap stock oil.
15 soap stock oil to methanol ratio, obtaining maximum FFA conversion (97.2%). Synthesized catalyst was also investigated for its reusability study. It has been found that catalyst can be used for three repetitive cycles without significant loss in their activity. There is also reducing cost for downstream processes like separation and recovery of unreacted alcohol that appears on the final cost of biodiesel. In short, a cost effective biodiesel can be produced by using a BBSA catalyzed process from soap stock oil.
Acknowledgements
Authors are very much thankful to the Applied Chemistry Department, SVNIT, Surat for laboratory facility and Council of Scientific and Industrial Research (CSIR), New Delhi, India (Sanction Order Letter No. 02(0170)/13/EMR-II) for financial assistance to this course. For structural characterization of synthesized products, we are very much thankful to Mr A. Narayanan, Indian Institute of Technology, Madras, Shree Dhanvantary Pharmaceutical analysis and Research Centre, Kim, Surat and Dr Anamik Shah, NFDD center, Department of Chemistry, Saurastra University, Rajkot India are gratefully acknowledged.References
- F. Ataya, M. A. Dubĕ and M. Ternan, Energy Fuels, 2008, 22, 679 CrossRef CAS.
- M. Canackci and J. V. Gerpen, Trans. ASAE, 2001, 44, 1429 Search PubMed.
- F. Ma and M. A. Hana, Bioresour. Technol., 1999, 70, 1 CrossRef CAS.
- M. Hayyan, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, Fuel Process. Technol., 2010, 91, 116 CrossRef CAS PubMed.
- M. Charoenchaitrakool and J. Thienmethangkoon, Fuel Process. Technol., 2011, 92, 112 CrossRef CAS PubMed.
- P. C. Jena, H. Raheman, G. V. Prasanna Kumar and R. Machavaram, Biomass Bioenergy, 2010, 34, 1108 CrossRef CAS PubMed.
- P. Nakpong and S. Wootthikanokkhan, Renewable Energy, 2010, 35, 1682 CrossRef CAS PubMed.
- G. Antolın, F. V. Tinaut, O. Y. Bricen, O. V. Castan, C. Perez and A. I. Ramrez, Bioresour. Technol., 2002, 83, 111 CrossRef.
- A. N. Phan and T. M. Phan, Fuel, 2008, 87, 3490 CrossRef CAS PubMed.
- B. Nebel and M. Mittelbach, Eur. J. Lipid Sci. Technol., 2006, 108, 398 CrossRef CAS PubMed.
- W. M. J. Achten, L. Verchot, Y. J. Franken, E. Mathijs, V. P. Singh and R. Aerts, Biomass Bioenergy, 2008, 32, 1063 CrossRef CAS PubMed.
- N. Foidl, G. Foidl, M. Sanchez, M. Mittelbach and S. Hackel, Bioresour. Technol., 1996, 58, 77 CrossRef CAS.
- M. L. Savaliya and B. Z. Dholakiya, Catal. Lett., 2014, 144, 1339 CrossRef.
- M. L. Savaliya and B. Z. Dholakiya, Appl. Catal., A, 2015, 494, 11 CrossRef PubMed.
- J. Yan and T. Lin, Appl. Energy, 2009, 86, 1 CrossRef PubMed.
- G. P. Hammond, S. Kallu and M. C. Mc Manus, Appl. Energy, 2008, 85, 506 CrossRef CAS PubMed.
- M. Canakc, Bioresour. Technol., 2008, 98, 183 CrossRef PubMed.
- J. J. Zhang and L. F. Jiang, Bioresour. Technol., 2008, 99, 8995 CrossRef CAS PubMed.
- M. L. Savaliya and B. Z. Dholakiya, Res. Chem. Intermed., 2015 DOI:10.1007/s11164-014-1885-1.
- Y. L. Wen, H. Z. Min and Q. D. Zhang, Bioresour. Technol., 2008, 99, 8752 CrossRef PubMed.
- N. Piyanuch and W. Sasiwimol, Renewable Energy, 2010, 35, 1682 CrossRef PubMed.
- G. Gauglitz and T. Vo-Dinh, Handbook of Spectroscopy, Wiley-VCH Verlag Gmbh & Co. Kgaa, Weinheim, 2003, ISBN 3-527-29782-0 Search PubMed.
- N. Tsubouchi, K. Xu and Y. Ohtsuka, Energy Fuels, 2003, 17, 1119 CrossRef CAS.
- Q. Shu, J. Gao, Z. Nawaz, Y. Liao, D. Wang and J. Wang, Appl. Energy, 2010, 87, 2589 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- X. Y. Liu, M. Huang, H. Long Ma, Z. Zhang, J. M. Gao, Y. L. Zhu, X. J. Han and X. Y. Guo, Molecules, 2010, 15, 7188 CrossRef CAS PubMed.
- E. L. Margelefsky, B. Anissa, R. K. Zeidan, V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2008, 130, 13442 CrossRef CAS PubMed.
- T. Dong, D. Gao, C. Miao, X. Yu, C. Degan, M. Garcia-Pérez, B. Rasco, S. S. Sablani and S. Chen, Energy Convers. Manage., 2015 DOI:10.1016/j.enconman.2015.06.072.
- J. K. M. Saunders and B. K. Hunter, Modern NMR Spectroscopy-a Guide for Chemists, Oxford University Press, Oxford, 2nd edn, 1993 Search PubMed.
- I. Wawera and S. Witkowski, Curr. Org. Chem., 2001, 5, 987 CrossRef.
- S. Fang and G. Yihang, Green Chem., 2014, 16, 2934 RSC.
- I. M. Lokman, U. Rashid, R. Yunus and Y. H. Taufiq-Yap, Catal. Rev.: Sci. Eng., 2014, 56, 187 CAS.
- N. Diamantopoulos, D. Panagiotaras and D. Nikolopoulos, J. Thermodyn. Catal., 2015, 6, 1 Search PubMed.
- Y. M. Sani, A. O. Raji, P. A. Alaba, A. R. Abdul aziz and W. M. A. Wan daud, BioResources, 2015, 10, 3393 CrossRef CAS.
- J. Alcañiz-Monge, G. Trautwein and J. P. Marco-Lozar, Appl. Catal., A, 2013, 468, 432 CrossRef PubMed.
- A. Takagaki, M. Toda, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Catal. Today, 2006, 116, 157 CrossRef CAS PubMed.
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS PubMed.
- W. Y. Lou, M. H. Zong and Z. Q. Duan, Bioresour. Technol., 2008, 99, 8752 CrossRef CAS PubMed.
- M. H. Zong, Z. Q. Duan, W. Y. Lou, T. J. Smith and H. Wu, Green Chem., 2007, 9, 434 RSC.
- V. L. Budarin, J. H. Clark, R. Luque and D. J. Macquarrie, Chem. Commun., 2007, 6, 634 RSC.
- T. Liu, Z. Li, W. Li, C. Shi and Y. Wang, Bioresour. Technol., 2013, 133, 618 CrossRef CAS PubMed.
- A. M. Dehkhoda, A. H. West and N. Ellis, Appl. Catal., A, 2010, 382, 197 CrossRef CAS PubMed.
- M. Zhang, A. Sun, Y. Meng, L. Wang, H. Jiang and G. Li, Catal. Surv. Asia, 2015, 19, 61 CrossRef CAS.
- U. Rashid, F. Anwar, B. R. Moser and G. Knothe, Bioresour. Technol., 2008, 99, 8175 CrossRef CAS PubMed.
- I. M. Rizwanul Fattah, H. Masjuki, A. Liaquat, R. Ramli, M. Kalam and V. Riazuddin, Renewable Sustainable Energy Rev., 2013, 18, 552 CrossRef PubMed.
- S. Y. No, Renewable Sustainable Energy Rev., 2011, 15, 131 CrossRef CAS PubMed.
- M. Tariq, S. Ali, F. Ahmad, M. Ahmad, M. Zafar, N. Khalid and M. A. Khan, Fuel Process. Technol., 2011, 92, 336 CrossRef CAS PubMed.
- P. Nair, Y. C. Sharma, B. Singh and S. N. Upadhyay, J. Cleaner Prod., 2012, 30, 82 CrossRef PubMed.
- Y. C. Sharma, B. Singh and S. N. Upadhyay, Fuel, 2008, 87, 2355 CrossRef CAS PubMed.
- R. Arora, V. Kapoor, and A. Pal Toor, 2nd International Conference on Emerging Trends in Engineering and Technology (ICETET'2014), London (UK), May 30–31, 2014, 196 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13422f |
| This journal is © The Royal Society of Chemistry 2015 |