Production of a non-cytotoxic bioflocculant by a bacterium utilizing a petroleum hydrocarbon source and its application in heavy metal removal
M. Pathaka,
A. Devi*a,
K. G. Bhattacharyyab,
H. K. Sarmac,
S. Subudhid and
B. Lald
aEnvironmental Chemistry Laboratory, Resource Management and Environment Section, Life Science Division, Institute of Advanced Study in Science and Technology, Guwahati, Assam 781035, India. E-mail: deviarundh2@yahoo.co.in; Fax: +91 3612273062; Tel: +91 3612912075 ext. 118
bDepartment of Chemistry, Gauhati University, Guwahati, Assam, India
cDepartment of Biotechnology, Gauhati University, Guwahati, Assam, India
dEnvironmental and Industrial Biotechnology Division, The Energy and Resources Institute, New Delhi, India
First published on 22nd July 2015
Abstract
A bacterium isolated from the activated sludge of an oil refinery of Assam, India retained efficient bioflocculating activity through production of the bioflocculant when it was grown on a crude oil amended medium void of any other carbon source. The bioflocculating activity gained from the optimized medium broth was 86.2%, which could be enhanced up to 89.1% with the purified bioflocculant. During the course of the bioflocculant production, the bacterium utilized about 77% of the petroleum hydrocarbons after incubation for 168 h when the activity was found to be the highest. The bioflocculant was efficient in flocculating Ni2+, Zn2+, Cd2+, Cu2+ and Pb2+. The bioflocculant was characterized as a glycoprotein complex by biochemical tests, FT-IR, SEM-EDX and LC/MS analyses. The bioflocculant showed negligible cytotoxicity on testing with the L292 cell line indicating the tremendous possibility of its use in bioremediation.
1 Introduction
In the field of bioremediation and bioprocesses, microbial involvements are receiving wide attention and the search for robust microorganisms with desirable results has been actively pursued. Microorganisms can produce surfactants, emulsifiers, enzymes, proteins and exopolysaccharides, which participate in bioremediation processes through both physical and biochemical mechanisms. Microbial flocculants are considered as a key asset in remediating water contaminated with inorganic and organic species through the process of “Bioflocculation” which is considered to be more efficient than the common chemical flocculation process.1 Several bacterial species like Alcaligenes latus, Paenibacillus sp., Bacillus sp., Pseudomonas aeruginosa, Rhodococcus sp., Acinetobacter sp. etc., are known for their capacity to generate bioflocculants. One notable advantage in the use of a bioflocculant is its innocuous nature to the ecosystem.2 Bioflocculants consist of microbial metabolic products that may include glycoproteins, polyoses, proteins, etc.3 In obtaining an effective bioflocculant, different media including brewery wastewater, soybean juice, fishmeal wastewater, etc., are in use.4The bioremediation of petroleum and petroleum based pollutants has been a prime concern during the last few decades and many pure and mixed bacterial consortia have undergone scientific scrutiny and evaluation for practical and systematic applications.5 Often, oil-degrading microorganisms produce extracellular surface-active products to enhance the utilization of oily substrates through increased oil solubility or dispersion. Pseudomonas aeruginosa has been a prime candidate in this regard and has been widely tested for in situ or ex situ degradation of petroleum hydrocarbons leaving behind beneficial or harmless metabolites.6 Many microorganisms cannot produce biosurfactants but are still able to degrade oil substrates effectively via the formation of extracellular or cell membrane-bound bio-emulsifiers such as exopolysaccharides (EPSs). The EPS-producing bacteria can utilize petroleum hydrocarbons as the sole carbon source for cell growth.7 On the other hand, microorganisms such as Rhodococcus are known to utilize n-hexadecane more efficiently at low temperatures through biosurfactant or bioflocculant production and in the process, can degrade linear and branched alkanes.8 Microbial cells may produce such extracellular substances in the form of capsules or mucoid secretions that may interact with hydrophobic substrates, such as hydrocarbons. Achromobacter sp. has also been reported recently as a potential source for bioflocculant production.9
The following work was designed to evaluate the appropriate and optimum conditions for the production of an efficient bioflocculant by a potent bacterial strain isolated from the activated sludge of the effluent treatment system in petroleum refineries thriving on petroleum crude oil and this is the first report of such an investigation. Further, the efficiency of such a bioflocculant was determined in vitro for possible use in removing heavy metals from water which was also proved to be a non-cytotoxic biopolymer.
2 Experimental
2.1 Collection of samples and chemicals
Activated sludge samples were collected from the effluent treatment plants of the oil refineries at Guwahati, Bongaigaon and Numaligarh, Assam, India. Petroleum crude oil was collected from Numaligarh Refinery Limited, Golaghat district, Assam, India. All the chemicals were purchased from HiMedia chemicals, Sigma-Aldrich, SRL India and MERCK, India.2.2 Isolation and screening of bioflocculant producing bacteria
Bioflocculant producing bacteria were isolated from the activated sludge samples following the methodology reported earlier through an enrichment culture technique and screened by a spread plate technique with selective media agar plates.10 The isolates were further grown in a production medium and their bioflocculating activities were determined by flocculating a kaolin suspension with the supernatants of the production medium broth as reported earlier by Kurane et al.11 1.0 litre of the production medium contained 5.0 g yeast extract, 5.0 g peptone, 2.0 g K2HPO4, 5.0 g KH2PO4, 1.0 g NH4Cl, 1% glycerol, 0.5 g MgSO4, 2.5 g NaCl, and 0.2% CaCO3. The initial pH of the medium was maintained at 7.2 and incubated for 72 h in an orbital shaker at 37 ± 2 °C with a rotation of 150 × g. 0.04 g of kaolin clay was suspended in 9.45 mL distilled water and 0.5 mL of 1% CaCl2 solution was mixed thoroughly with the kaolin suspension. 0.05 mL of the culture supernatant (taken as the raw bioflocculant) was added to the mixture and the pH of the mixture was adjusted to 7.0. The mixture was vortexed in a test tube for 1 min and then was kept at room temperature for 5 min. The flocculating activity was calculated using the formula,| Bioflocculating activity = ((As − A550)/As) × 100% |
2.3 Production and purification of the bacterial bioflocculant
The bioflocculant produced by the selected bacteria, Pseudomonas aeruginosa IASST201, was extracted from the bacteria-grown production medium broth (crude oil as carbon source) when the bioflocculating activity was found to be at its highest, by pouring it onto two volumes of ice-cold ethanol at 4 °C and keeping it for 12 h to separate the bioflocculant content. The resulting precipitate was collected by centrifugation at 6000 × g for 30 min. The bioflocculant sample was then lyophilized and solubilized with deionized water (at concentration, 10 mg mL−1) and purified with a column packed with DEAE-cellulose-52 (26 mm × 150 mm). The purified bioflocculant was eluted with deionized water and (0.1–1 M) NaCl in a phosphate buffer (pH 7) with a flow rate of 0.5 mL min−1.12 The optimum dose of the purified bioflocculant to obtain the highest flocculating activity was determined as described earlier by taking a sample of 10–150 μg. To retain the exopolysaccharide (EPS) content of the bioflocculant for LC/MS analysis, the protein content was separated with a chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyl alcohol (5
butyl alcohol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extraction mixture. The material was then dialyzed against de-ionized water overnight and vacuum-dried to obtain the purified EPS of the bioflocculant.13
1) extraction mixture. The material was then dialyzed against de-ionized water overnight and vacuum-dried to obtain the purified EPS of the bioflocculant.13
2.4 Degradation of crude oil hydrocarbons
In order to determine the utilization of petroleum hydrocarbons in the production of the bioflocculant by the bacteria, the residue of the optimized production medium broth was extracted (v/v) with dichloromethane (DCM) when the bioflocculating activity was found to be at its highest.14 The treated portion along with an untreated control was subjected to FT-IR analysis (NICOLET 6700 FTIR-Spectrophotometer, USA). The DCM extracted portions of crude oil (control) and degraded oil (test) were analyzed though a triple quadruple Gas Chromatograph-Mass Spectrometer (GC/MS TQ8030, Shimadzu, Japan) with an autoinjector (AOC 20I, GC-2010, E). The GC program was optimized for the detection of petroleum hydrocarbons and all analyses were carried out with the split ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Helium was used as the carrier gas with a flow rate of 1.0 mL min−1. The injection temperature was set at 300 °C. The column oven temperature was set at 60 °C with a hold time of 5 min and was subsequently increased to 300 °C with a ramp of 10 °C min−1. The final hold was for 44 min. The ion-source temperature was set at 230 °C for MS using an interface temperature of 310 °C. The mass range (m/z) was selected as 45–800 for the entire analysis. The chromatograms were analyzed with GC-MS solution software (version 4). For compound identification, the NIST 11 library database was used. The percentage degradation of the hydrocarbons was calculated by the formula, [(mHc − mH)/mHc] × 100, where mHc and mH are the sum of the total areas of the peaks for the control sample and the test sample respectively.
1. Helium was used as the carrier gas with a flow rate of 1.0 mL min−1. The injection temperature was set at 300 °C. The column oven temperature was set at 60 °C with a hold time of 5 min and was subsequently increased to 300 °C with a ramp of 10 °C min−1. The final hold was for 44 min. The ion-source temperature was set at 230 °C for MS using an interface temperature of 310 °C. The mass range (m/z) was selected as 45–800 for the entire analysis. The chromatograms were analyzed with GC-MS solution software (version 4). For compound identification, the NIST 11 library database was used. The percentage degradation of the hydrocarbons was calculated by the formula, [(mHc − mH)/mHc] × 100, where mHc and mH are the sum of the total areas of the peaks for the control sample and the test sample respectively.
2.5 SEM and EDX study of the bioflocculant
The purified bioflocculant from the DEAE column was powdered with a lyophilizer (Thermo modulo D, USA) and was subjected to Field Emission Scanning Electron Microscopy (FE-SEM). The dry bioflocculant was mounted on a stub over adhesive tape and was coated with gold–palladium powder using a Sputter Coater (SC-7625, EMITECH, India) before finally attaching the stub over the microscope support. Scanning electron microscopic images were taken at 5 kV with a FE-SEM (Zeiss, ∑-Sigma, Germany) Scanning Electron Microscope. The energy dispersive X-ray analysis (EDX) measurements were performed with an X-ray detector and were analyzed with INCA 4.15 EDS software (Oxford Instruments).2.6 Characterization of the bioflocculant
The bioflocculant composition of the column purified sample was determined with respect to polysaccharide and protein content. The protein concentration of the bioflocculant was measured using the Bradford method while the total carbohydrate content was determined with the anthrone method.15,16 The DNS reaction, carbazol–sulfuric acid and Elson–Morgan assays were used to determine the contents of the reducing sugars, uronic acids and amino sugars respectively.17 The purified bioflocculant was subjected to FT-IR in ATR mode within a range of 500 to 4000 cm−1. For the characterization of the bioflocculant for its monosaccharide composition, 10 mg of the purified EPS sample was hydrolyzed at 100 °C (6 h) with 2 N trifluoroacetic acid (TFA) in a hydrolysis tube.18 Excessive TFA was removed by vacuum evaporation. Denatured protein debris was precipitated by the addition of 5 mL of 80% (w/v) trichloroacetic acid, followed by incubation in ice for 30 min and further centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min at 4 °C). EPS was precipitated from the supernatant by the addition of ice-cold ethanol and the pellets were collected through further centrifugation (10
000 × g for 30 min at 4 °C). EPS was precipitated from the supernatant by the addition of ice-cold ethanol and the pellets were collected through further centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min at 4 °C). Determinative tests such as Benedict’s reaction, Seliwanoff’s test, the pyrocatechol reaction, the ketose test and Bial’s test were performed for characterizing the monomers of the EPS. The hydrolysate was precipitated and reconstituted with 1 mL of acetonitrile/water (80
000 × g for 30 min at 4 °C). Determinative tests such as Benedict’s reaction, Seliwanoff’s test, the pyrocatechol reaction, the ketose test and Bial’s test were performed for characterizing the monomers of the EPS. The hydrolysate was precipitated and reconstituted with 1 mL of acetonitrile/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v). The EPS fraction was separated and analyzed for different structural analogues by liquid chromatography–mass spectrometry (LC/MS, 1260 Infinity LC and 6410 Triple Quadrupole MS, Agilent Technologies, USA). A 2 μL sample aliquot was injected into a ZORBAX C18 column (2.1 × 50 mm2) using a gradient of water + 0.1% formic acid (solvent A) and acetonitrile (solvent B) at 40 °C at a flow rate of 0.2 mL min−1 with a linear increase from 10 to 90% solvent B addition within 25 min, a modification of the method by François et al.19 ESI-MS spectra were obtained in positive ion mode and were analyzed using Agilent ChemStation Software. Full scan data were obtained by scanning from an m/z ratio of 50–950 with a fragmentor voltage calibrated at 135.0 V.
20 v/v). The EPS fraction was separated and analyzed for different structural analogues by liquid chromatography–mass spectrometry (LC/MS, 1260 Infinity LC and 6410 Triple Quadrupole MS, Agilent Technologies, USA). A 2 μL sample aliquot was injected into a ZORBAX C18 column (2.1 × 50 mm2) using a gradient of water + 0.1% formic acid (solvent A) and acetonitrile (solvent B) at 40 °C at a flow rate of 0.2 mL min−1 with a linear increase from 10 to 90% solvent B addition within 25 min, a modification of the method by François et al.19 ESI-MS spectra were obtained in positive ion mode and were analyzed using Agilent ChemStation Software. Full scan data were obtained by scanning from an m/z ratio of 50–950 with a fragmentor voltage calibrated at 135.0 V.
2.7 Study of heavy metal removal efficiency by bacterial bioflocculant
The heavy metal removal efficiency of the bacterial bioflocculant was determined using heavy metal solutions without kaolin clay. For this purpose, aqueous solutions containing Ni2+, Zn2+, Cd2+, Cu2+ and Pb2+ at concentrations of 10 mg L−1 each were prepared. The bioflocculation experiment was performed with both the raw bacterial culture supernatant and the purified bioflocculant as per the methodology mentioned in Section 2.1. The samples were maintained at pH 7 and the final volume was made up to 10 mL. After the bioflocculant was added, the samples were vortexed and kept at rest for 5 min. The metal concentrations in the upper layer of the solution (3 mL) were measured with an Atomic Absorption Spectrometer (Shimadzu AA7000, Japan) as well.2.8 Cytotoxicity study of the bioflocculant
For determining the toxicity level of the bioflocculant, a 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye conversion assay was carried out on a mouse L929 fibroblastic cell line (obtained from NCCS, Pune).20 The L929 cells were cultured at a density of 1 × 104 cells per well in a 100 μL volume of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum in a 96-well cell culture plate. The MTT assay is a colorimetric cytotoxicity and proliferation detection assay, based on the metabolic activity of viable cells in reducing tetrazolium salts (MTT).21After 24 h, the cultured cells were treated with a series of different doses (10, 30, 50, 70, 90, 110, 130 and 150 μg) of column purified bioflocculant in 100 μL DMEM without serum per well plate and were incubated for a further 24 h. This was followed by the removal of the medium and treatment with MTT dye at a final concentration of 0.5 mg mL−1and further incubation for 4 h. Finally, 100 μL of dimethylsulfoxide (DMSO) was added to each well to dissolve the blue formazan precipitate, and the absorbance was measured at 570 nm using a microplate reader (Bio-Rad Model 680; Bio-Rad). The cell viability was expressed as a percentage with a control using the following equation,| Viability (%) = Nt/Nc × 100 |
2.9 Statistical analysis
All the experiments were performed in triplicates and the error bars in the figures represent the standard deviations of the data. The software Origin (version 8.5) was used for developing graphs supporting the experiments. Petroleum hydrocarbons, derivatives in crude oil and degraded compounds of crude oil found after utilization by the selected bacteria, were detected with GC/MS analysis and were depicted by a Venn diagram with the help of Vennture software (NIA Bioinformatics Software).223 Results and discussions
3.1 Isolation of the bioflocculant producing bacteria
Innumerable bioflocculant producing microorganisms have been isolated from oil contaminated soils and activated sludge with profound biological importance.23 The present study was therefore an attempt to characterize bioflocculant producing bacteria from activated sludge produced in effluent treatment plants in oil refineries. The perspective behind this was to generate a significant achievement that can be extended for large scale effluent treatment in oil refineries worldwide. The bacteria isolated from such sites usually have the potential for surviving in toxic and extreme environments. When the most prominent isolates out of the selective 37 bioflocculant producers were subjected to a crude oil amended medium without any other carbon source, a bacterium, IASST201, was found to exhibit the most efficient bioflocculating activity and was therefore chosen for further experiments. The molecular identity of the isolate was established as Pseudomonas aeruginosa strain IASST201 through 16s rRNA sequencing. The generated 16s rRNA gene sequences were submitted to genbank with an accession number of![[K with combining low line]](https://www.rsc.org/images/entities/char_004b_0332.gif)
![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) .10
.10
3.2 Production of bioflocculant and the study of flocculation pattern
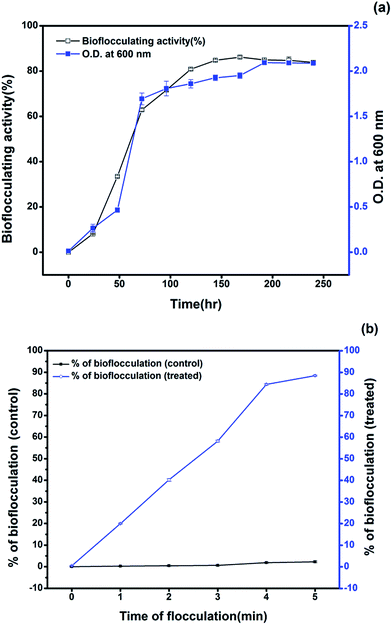
As for routine analyses, the selected strain showed 78.8% efficiency in flocculating the kaolin suspension in the initial production medium amended with 1% crude oil as the sole carbon source. The optimum crude oil concentration was found to be 2% to get enhanced bioflocculating activity of the selected isolate in the production medium. Several workers have reported and studied the capacity of bioflocculant producing bacteria in utilizing uncommon sources as nutrients for better production of efficient bioflocculants. A bacterial species, Rhodococcus erythropolis, was targeted to utilize a cheap medium composed of sludge and livestock wastewater for obtaining an active bioflocculant with a yield of 1.6 g L−1 after 72 h and an activity of 83.6% with the raw supernatant.24 In the present case, the bacterial growth curve obtained from the optimized production medium indicated the emergence of a stationary phase after 192 h of incubation (Fig. 1a) and the yield of bioflocculant was found to be 1.48 g L−1 with an activity of 86.2 ± 0.51%. Study of the growth rate and the bioflocculating activity in the production medium showed a maximum activity after 168 h of inoculation with the formation of clumps or aggregating flocs of crude oil at around the 36th hour of incubation. The comparatively longer time required for showing the maximum bioflocculating activity may be attributed to the use of petroleum crude as the carbon source since the Pseudomonas species shows maximum growth behaviour in the presence of glucose as the sole carbon source. Utilization of petroleum hydrocarbons by bacteria as a carbon source for producing extracellular metabolites has not been reported much and the pattern of growth vs. the production has been found to vary in various cases. However, it has been known that certain species of bacteria produce exopolysaccharides during the hydrocarbon degradation process.25 The flocculation of the kaolin suspension varied from 20.0, 40.2, 58.2, 82.5 to 86.2% after the flocculant was applied for a time period ranging from 1 to 5 min with an interval of 1 min respectively (Fig. 1b).3.3 Utilization of the petroleum hydrocarbons during bioflocculant production
Comparison of the FTIR spectra of the control (original crude oil sample) and the test sample (crude oil sample used as the carbon source for bioflocculant production by the bacteria) clearly indicates the persistence of a degradation process. The control and the test sample show the presence of aromatic C–H, substituted aromatic ring, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C stretch, CH3 and CH2 (750 cm−1, 812 cm−1, 878 cm−1, 1455 cm−1, 1598 cm−1, 2930–2850 cm−1 and 2960–2870 cm−1 respectively). However, two bands around 1602 and 1697 cm−1 indicating carbonyl C
C, C–C stretch, CH3 and CH2 (750 cm−1, 812 cm−1, 878 cm−1, 1455 cm−1, 1598 cm−1, 2930–2850 cm−1 and 2960–2870 cm−1 respectively). However, two bands around 1602 and 1697 cm−1 indicating carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the control sample disappear in the test sample, which, instead, has a strong absorption band at 1673 cm−1 for –C
O stretching in the control sample disappear in the test sample, which, instead, has a strong absorption band at 1673 cm−1 for –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretching or a
C– stretching or a ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group indicating the possible presence of a ketone or carboxylic acid formed as a degradation intermediate. Interestingly, the triplet of 750, 812 and 878 cm−1 has a lower intensity in the degraded sample. A small but expanded band around 3265 cm−1 in the test sample indicates –OH stretching bands, which might result from alcohols and acids produced due to the mineralization of the aliphatic and aromatic components of crude oil during the microbial action26 (Fig. 2).
O group indicating the possible presence of a ketone or carboxylic acid formed as a degradation intermediate. Interestingly, the triplet of 750, 812 and 878 cm−1 has a lower intensity in the degraded sample. A small but expanded band around 3265 cm−1 in the test sample indicates –OH stretching bands, which might result from alcohols and acids produced due to the mineralization of the aliphatic and aromatic components of crude oil during the microbial action26 (Fig. 2).
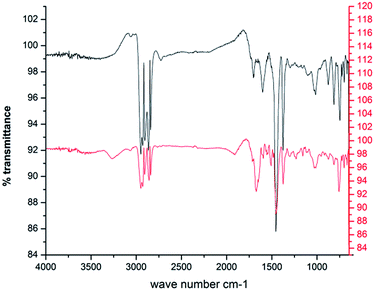 | ||
Fig. 2 FTIR spectra of the DCM extracted portion of crude oil ( ) and the degraded crude oil ( ) and the degraded crude oil ( ) during the production of the bioflocculant. ) during the production of the bioflocculant. | ||
A number of bacteria have been reported to degrade alkanes and aromatics as a source of carbon and energy. At the cost of utilizing such nutrients of hydrophobic nature, bacteria often release extracellular polymeric substances including surfactants and exopolysaccharides which help these microbes either to make the hydrophobic moiety of the substrate available to a hydrophilic one or by adhering to the hydrophobic surface and leading to the formation of an emulsion.25 Though surfactants are commonly known for their participation in making the hydrophobic substrates available for microbial utilization, several investigations indicate a positive correlation between exo-polymer (such as bioflocculant) production and microbial degradation of hydrocarbons. Researchers hypothesized depending on their experiments on EPS producing bacteria that the formation of oil aggregates is contributed to by the production of EPS, which also contributes to the fate of the oil by influencing the dissolution, bioavailability and ultimate degradation of hydrocarbons by indigenous oil degrading communities. In particular, polycyclic aromatic hydrocarbons (PAHs) are poorly soluble and generally less amenable to biodegradation compared to their aliphatic counterparts. To circumvent the limitations in hydrocarbon bioavailability, some microorganisms produce exopolysaccharides as a mechanism to increase the bioavailability of these compounds for biodegradation.27 During the utilization or degradation of petroleum based compounds by microbes, substrates are often converted into alcoholic, ester or acid derivatives. The mode of the bacterial action in degrading petroleum hydrocarbons and producing the bioflocculant in this course which was later applied in heavy metal removal is presented here within a schematic diagram in Fig. 3.
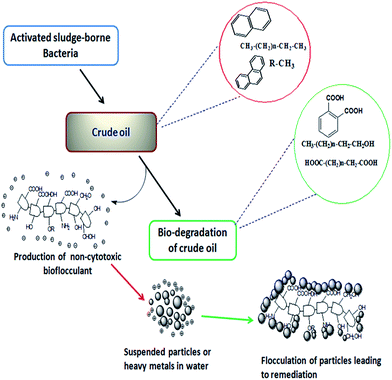 | ||
| Fig. 3 Schematic presentation showing the bacterial action for the bioconversion of petroleum hydrocarbons through degradation along with the production of active biopolymers. | ||
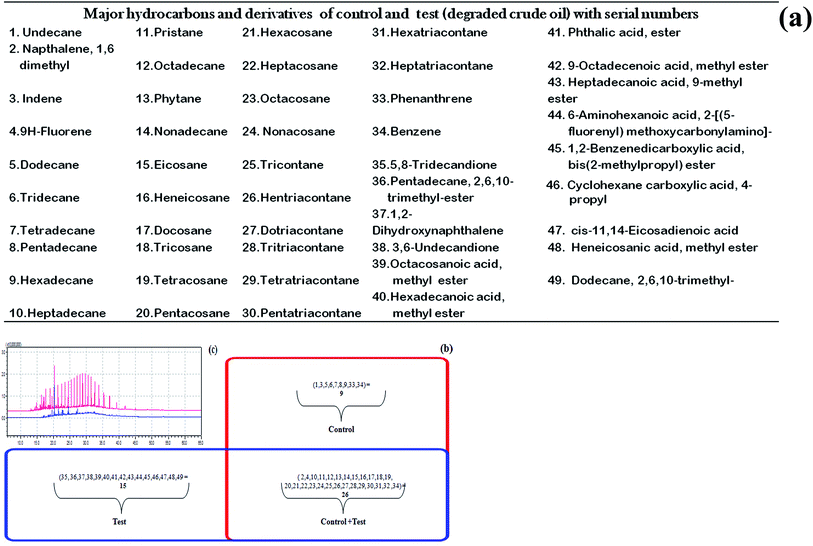
In microbial actions, the biodegradation of aliphatics is initiated by the oxidation of a terminal methyl group to a primary alcohol, which gets further oxidized to the corresponding aldehyde, and finally to the fatty acid. However, in some cases, both ends of the alkane molecule may be involved in the oxidation process thereby producing ω-hydroxy fatty acids, which are further converted to dicarboxylic acids by β-oxidation.28 Sub-terminal oxidation of n-alkanes has also been reported earlier.29 The product generates a secondary alcohol which is converted to the corresponding ketone, and then oxidized by Baeyer–Villiger monooxygenase to an ester. The ester is hydrolyzed by an esterase, generating an alcohol and a fatty acid. Both terminal and sub-terminal oxidation can co-exist in the action of some microorganisms. In the present study, the degradation of crude oil was around 77% when bioflocculating activity was at its maximum. The GC/MS analysis of the control crude oil sample generated the identity of 4 major polycyclic aromatic hydrocarbons (PAH) (viz., naphthalene, indene, phenanthrene and fluorene) along with benzene and 29 aliphatic hydrocarbons (C11 to C37) that include pristane (C19) and phytane (C20). The test crude oil sample also showed the presence of two PAHs (naphthalene and fluorene), some benzene derivatives and 23 aliphatic hydrocarbons (C17 to C37) along with pristane and phytane, and 15 prominent degradation intermediates forming different esters and acids (phthalic acid ester, hexadecanoic acid ester, etc.). The control and the test samples had 26 constituents in common out of the identified 49 constituents including degraded and non-degraded intermediates. The distribution of these components is tabulated and shown in a Venn diagram with a comparative GC/MS chromatogram (Fig. 4a–c).
3.4 Chemical characterization, morphology and element composition of bioflocculant
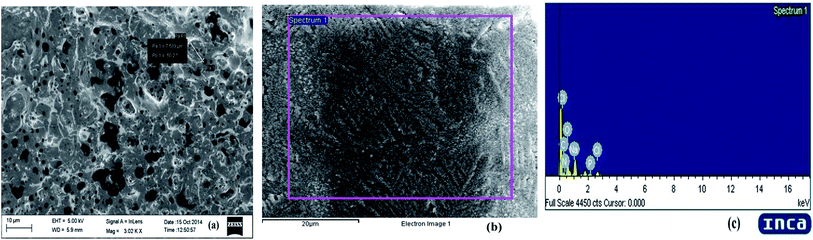
Bioflocculants have been reported to consist of proteins, glycoproteins, polysaccharides, lipids and glycolipids. In the case of a bioflocculant produced by the P. aeruginosa strain, chemical analysis of the biopolymer revealed it to be a sugar protein derivative, composed of protein and carbohydrate including neutral sugars, uronic acid and amino sugars as the principal constituents.30 The biochemical tests conducted on the purified bioflocculant sample obtained from our selected bacterium confirmed the presence of around 85.1% total carbohydrate but a low protein content of 8.2% indicating the glycoprotein nature of the bioflocculant. The carbohydrate part consisted of neutral sugars, amino sugars and uronic acid in the ratio 80.5, 4.4 and 0.2% respectively. Benedict’s and DNS tests confirmed the presence of reducing sugars in the bioflocculant while Seliwanoff’s reaction showed the absence of fructose. The presence of xylose and glucose were also recorded from the ketose test, orcinol test and pyrocatechol reaction.The SEM image (Fig. 5a) of the bioflocculant indicated a crystalline and irregular shaped morphology of the purified bioflocculant. The SEM-EDX analysis of the dried purified bioflocculant sample showed the presence of carbon, nitrogen, oxygen, sodium, phosphorus and chlorine in the proportions of 25.30, 13.91, 48.85, 10.22, 0.33 and 1.39% in the scanned area (Fig. 5b and c). The abundance of carbon, nitrogen and oxygen further proved the elemental characteristics of the bioflocculant as a carbohydrate–protein complex moiety.
3.5 FTIR and LC/MS analysis of bioflocculant
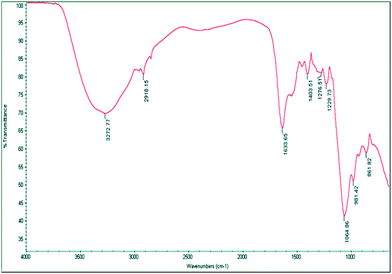
The FTIR spectrum in the ATR mode of the purified bioflocculant has a broad absorption band at 3272.77 cm−1, assigned to –OH or –NH vibrations. An asymmetrical band at 1633.65 cm−1 could be attributed to –NH or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from –NHCOCH3 groups and a weak band at 1403.51 cm−1, to symmetrical and asymmetrical stretching of the C
O stretching vibrations from –NHCOCH3 groups and a weak band at 1403.51 cm−1, to symmetrical and asymmetrical stretching of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the carbohydrate in the bioflocculant. A small band around 2918.15 cm−1 points to the C–H stretching vibrations of –CH2 groups while strong bands at 1052.87 and 1064.86 cm−1 could be attributed to asymmetrical stretching of the C–O–C ester linkage. The presence of β-glycosidic linkages between the sugar monomers is indicated by a small absorption band at 861.82 cm−1 (Fig. 6). The functional groups in the polymer molecule are important determinants for the flocculating activity. The –OH, –COOH, COO– groups in the bioflocculant and H+, OH− groups on the surface of the suspended particles may form hydrogen bonds when the bioflocculant chains approach the surface of particles.31
O groups of the carbohydrate in the bioflocculant. A small band around 2918.15 cm−1 points to the C–H stretching vibrations of –CH2 groups while strong bands at 1052.87 and 1064.86 cm−1 could be attributed to asymmetrical stretching of the C–O–C ester linkage. The presence of β-glycosidic linkages between the sugar monomers is indicated by a small absorption band at 861.82 cm−1 (Fig. 6). The functional groups in the polymer molecule are important determinants for the flocculating activity. The –OH, –COOH, COO– groups in the bioflocculant and H+, OH− groups on the surface of the suspended particles may form hydrogen bonds when the bioflocculant chains approach the surface of particles.31
The anionic nature of the bioflocculant is established by the detection of carboxyl, hydroxyl and amine groups in the FT-IR spectra. These groups are likely to serve as the binding sites for divalent cations and suspended particles in a solution.32 These functional groups in the bioflocculant have their origin in the sugars and proteins and could be the sites for the bioflocculant activity.
The LC/MS study of the purified bioflocculant gave the proper idea about the monomer constituents of the exopolysaccharide composition. In the LC/MS data based investigation the characterization of exopolysaccharides was focused, as bioflocculants were investigated as nothing but a composition of extracellular or surface polysaccharides in a combination with outer-membrane proteins or lipid moieties. The exopolysaccharides play a principal role in the formation of cell aggregates, the initiation of flocculation and similar processes. This property is vital for wastewater treatment and particle aggregation.33 Hino et al.7 first investigated EPS-producing bacteria that could utilize petroleum hydrocarbons as the sole carbon source for cell growth. They noted that exopolysaccharides play central roles in the formation of biofilms, and coined the term “slime” which signified that the “slime-producing bacteria” could be utilizers of petroleum hydrocarbons. These biofilms are also known as a mass of extracellular components often bearing cell-adhesion or attaching species to suspended particles.
Precisely, the constituents of bacterial exopolysaccharides often share in the compositions of bioflocculants. Bacterial EPSs were reported as a broad range of non-volatile sugar compositions such as glucose, rhamnose, galactose, mannose, xylose, n-acetyl galactosamine, n-acetyl fucosamine, n-acetyl glucosamine, mannuronic acid etc.34 The LC/MS analysis for the detection of the sugar monomer composition of the bacterial bioflocculant revealed the existence of glucose (Glu), xylose (Xyl) and n-acetyl hexosamine (HexNac) in the EPS portion through the ESI mass spectrum.35
LC/MS-ESI is a suitable interface for MS of polar and thermally labile compounds which includes the detection of non-volatile sugars, proteins etc. In the present case, Na+ and H+ ion adducts have been identified for the sugar monomers of the sample depending on fragmentation and elution time. Sodium adduct ions are formed for glucose as (Glu + Na)+ (m/z 203.1) and (Glu + Glu + Na)+ (m/z 383.1). The m/z of 244.2 represents n-acetyl hexosamine forming a sodium adduct (HexNac + Na)+. The presence of xylose could be predicted from the formation of both proton and sodium adducts with m/z of 151.4 (Xyl + H)+ and 325.0 (Xyl + Xyl + Na)+ respectively (Fig. 7a and b).
These results along with the functional groups deduced from the FTIR analysis played a pivotal role in the prediction the bioflocculant as a polymer consisting of glucose, xylose and n-acetyl hexosamine with a small protein content.
3.6 Dose optimization of purified bioflocculant and cytotoxicity test
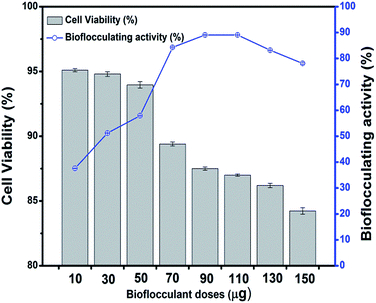
The purified bioflocculant was found to be more effective when optimized in dose, where it showed a constant flocculating activity of 89.1 ± 0.08% when 90 to 110 μg of it was employed in kaolin precipitation assay. Bacterial metabolites often cause harm to the host leading to epidemic diseases along with neurotoxic and cytotoxic effects. However, in the present case, the bioflocculant was not seen to exhibit any such harmful activities. There are reports of the non-toxic effect of bioflocculants obtained from a Klebsiella species in mammals in terms of external clinical symptoms.36,37 Interestingly, Pseudomonas aeruginosa species are known as notorious producers of toxic substances like exotoxins, but the utilization of such extracellularly produced flocculants need not necessarily establish them as hazardous biological materials. The application of the bioflocculant obtained from the selected strain in this work showed no toxic effect on the mouse fibroblast L292 cell line and produced a bench-mark as a non-cytotoxic glycoprotein-like polymer which could be used as a possible biological material, for uses including biological interfaces. The cells of this mouse fibroblastic cell-line were found to be viable through a MTT dye conversion assay ranging from 95.1 to 84.2% when 10 to 150 μg of the purified bioflocculant was subjected to the micro-titer plate wells (Fig. 8). This proves the probable utility of this bioflocculant encompassing safety standards for animals and humans and therefore may be considered as a ‘novel compound’.3.7 Application of bioflocculant for heavy metal removal
Bioflocculants, like extracellular polysaccharides often play an important role in controlling heavy metal pollution in the sewage treatment process and they are recommended as surface-active agents for the removal of heavy metals. Bacterial species from the genera Pseudomonas, Bacillus, Herbaspirillum, Paenibacillus, etc. are always being mentioned as good bioflocculant producers and their application in heavy metal removal has been studied. Due to the extensive capacity of bioflocculants for metals, they are recommended as surface-active agents for the removal of heavy metals. The appropriate physical and chemical properties, the availability of appropriate binding sites and the tertiary structure of the bioflocculant may all contribute to the metal-binding interactions. The efficiency of bioflocculation depends on the conformation of the polymer with the adsorbed ions.38 In this investigation, biochemical tests, FT-IR and LC/MS analyses have established the structure of the bioflocculant as consisting of sugar-protein, with –OH, and –C–O–C linkages which could be the possible sites for metal binding and chelation through chemical interactions or siderophore expression. These groups, together with the amino group, could serve as a binding site for metal ions which enhances the flocculating activity of the bioflocculant by bridging between it and the suspended particles in solution, and are likely to be the preferred groups for the process of adsorption.39 Explaining the flocculation mechanism, Wu and Ye suggest that the cations (from CaCl2) are effectors in the action of a bioflocculant polymer by neutralizing and stabilizing the residual negative charge of the functional groups by forming bridges between particles.3 The DCB (Divalent Cation Bridge) theory, the most convenient one in explaining the mechanism of flocculation, states that divalent cations bridge negatively charged functional groups within the EPS and this bridging helps to aggregate and stabilize the matrix of the biopolymer and microbes and therefore promotes bioflocculation.40 In general, bioflocculants have been found to have a net negative charge and the cation source (CaCl2 in the present case), by giving a positive charge to the heavy metals, opens the provision for the bioflocculant polymer to undergo ion interactions leading to formation of the floc. The presence of the acid moiety of a polysaccharide which was found through biochemical characterization of the present bioflocculant could also assist in metal uptake. Carboxylic groups present in exopolysaccharides work as a non-specific ion exchange material, which may convey chelating properties. The protein constituents of a glycoprotein-like flocculant containing multiple carboxyl groups are also important in the process of flocculation with heavy metals. The efficiency to reduce the surface charge density by adsorption of the bioflocculant and the particles such as heavy metals becomes crucial.30In the present study, when the raw culture supernatant was applied as a bioflocculant, it was found to remove the heavy metals Ni2+, Zn2+, Cd2+, Cu2+, Pb2+ by 72.22 ± 0.22, 55.42 ± 0.31, 50.10 ± 0.13, 36.19 ± 0.08, 33.68 ± 0.16% respectively. Compared to this, the purified bioflocculant (with optimum dose) was found to be capable of removing Ni2+, Zn2+, Cd2+, Cu2+ and Pb2+ by 76.14 ± 0.11, 62.69 ± 0.48, 53.22 ± 0.04, 47.64 ± 0.47 and 40.58 ± 0.28% respectively. This signifies the efficiency of this bacterial bioflocculant in removing the chosen heavy metals in a pattern of Ni2+ > Zn2+ > Cd2+ > Cu2+ > Pb2+ in both the cases of the supernatant and the purified component (Fig. 9).
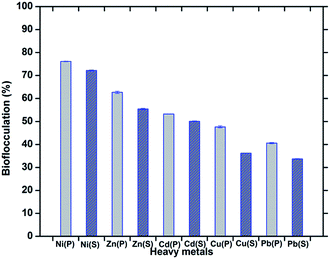 | ||
| Fig. 9 Bioflocculation of heavy metals by treatment with the purified bioflocculant (P) and the raw culture supernatant (S). The error bars represent the standard deviation of the data. | ||
The pH of the samples remained unaffected by the addition of the polymeric bioflocculant in its optimum dose and the highest activity of the bioflocculant was found at around pH 7 of the sample solution. The heavy metal removal capacity of a bioflocculant in an aqueous system is reported to be lower at low and high pH values of the system; at low pH, a high concentration of protons competes for the anionic sites on the polymer preventing the removal of the divalent cations. Thus, divalent cation binding is low. As the pH increases to its optimum value, which differs from one metal ion to another, the adsorbing surface is saturated with negative charges, resulting in an increased efficiency to bind and adsorb metal cations. At a pH higher than the optimum value, hydroxo species of the metals can be formed that do not bind to the adsorption sites on the surface of the adsorbent.41 The stability of pH obtained in the present study could be advantageous in removing other heavy metals and pollutants too from various waste water streams by bioflocculants in required doses.
4 Conclusions
The present work has identified a bacterium and further enumerated an efficient bioflocculant production system which has been optimized to an extent whereby the microorganism, that thrives on petroleum crude oil and is known for its hazardous effects towards the environment, could be utilized for enormous benefits in the study of producing effective microbial biopolymers through the course of utilizing such a source of energy. The bioflocculant obtained from these experiments has an excellent flocculating capacity of 86.2 ± 0.51% which could significantly remove 5 different heavy metals from aqueous solutions with a pattern of Ni2+ > Zn2+ > Cd2+ > Cu2+ > Pb2+, and Ni2+ as the highest by 76.14%. Experiments in support of characterizing this biopolymer, conclude the nature of it as a glycoprotein-like substance including glucose, xylose and n-acetyl hexosamine as the constituents of the exopolysaccharide part of the bioflocculant, which was produced during the utilization of hydrocarbons of crude petroleum oil by the selected bacterium. The bioflocculant was found to be sufficiently non-cytotoxic to the mammalian cell line even after application of it in the optimum dose necessary for showing the highest flocculating activity. This study is likely to lead to a system of symbiosis reflecting the microbial degradation–flocculation phenomena as a means to treat industrial effluents and trials for the production and characterization of more effective microbial bioflocculants may help other researchers to grab a thread leading to the idea of employing the microbial degradation and flocculation mechanism to treat various natural resources contaminated with industrial and anthropogenic activities.Acknowledgements
The authors are grateful to the Department of Biotechnology, Govt. of India, for financial support for the fulfilment of this work. We are also obliged to thank the Director of the Institute of Advanced Study in Science and Technology (IASST), Guwahati and the Director-General of The Energy and Resources Institute (TERI), New Delhi. We are thankful to the Guwahati Biotech Park, Govt. of Assam, India for the LC/MS analyses of the samples.References
- W. Chun, Z. Xin-Qing, G. Suo-Lian, M. Asraful Alam and B. Feng Wu, Bioresour. Technol., 2013, 135, 207–212 CrossRef PubMed.
- S. Dong, N. Ren, A. Wang, F. Ma and D. Zhou, J. Water Resour. Prot., 2008, 1, 1–65 Search PubMed.
- J. Y. Wu and H. F. Ye, Process Biochem., 2007, 42, 1114–1123 CrossRef CAS PubMed.
- Z. Zhang, B. Lin, S. Xia, X. Wang and A. Yang, J. Environ. Sci., 2007, 19, 667–673 CrossRef CAS.
- V. S. Cerqueira, E. B. Hollenbach, F. Maboni, M. H. Vainstein, F. A. O. Camargo, M. C. R. Peralba and F. M. Bento, Bioresour. Technol., 2011, 102, 11003–11010 CrossRef CAS PubMed.
- T. N. Gamble, M. R. Betlach and J. M. Tiedje, Appl. Environ. Microbiol., 1977, 33, 926–939 CAS.
- S. Hino, K. Watanabe and N. Tatkahashi, J. Ferment. Bioeng., 1997, 84, 528–531 CrossRef CAS.
- L. A. Sayavedra-Soto, W. N. Chang, T. K. Lin, C. L. Ho and H. S. Liu, Biotechnol. Prog., 2006, 22, 1368–1373 CrossRef CAS PubMed.
- S. Subudhi, N. Batta, M. Pathak, V. Bisht, A. Devi, B. Lal and B. A. Khulifah, Chemosphere, 2014, 113, 116–124 CrossRef CAS PubMed.
- M. Pathak, A. Devi, H. K. Sarma and B. Lal, J. Basic Microbiol., 2014, 54, 1–12 CrossRef PubMed.
- R. Kurane, K. Takeda and T. Suzuki, Agric. Biol. Chem., 1986, 50, 2301–2307 CrossRef CAS.
- K. Wang, W. Li, X. Rui, X. Chen, M. Jiang and M. Dong, Int. J. Biol. Macromol., 2014, 63, 133–139 CrossRef CAS PubMed.
- S. Cosa, A. M. Ugbenyen, L. V. Mabinya, K. Rumbold and A. I. Okoh, Environ. Technol., 2013, 34, 2671–2679 CrossRef CAS PubMed.
- E. E. Esin, S. Fikrettin and K. Ayten, Afr. J. Biotechnol., 2011, 10, 600–607 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- P. Gerhardt, R. G. E. Murray, W. A. Wood and N. R. Krieg, Methods for General and Molecular Bacteriology, ASM, Washington, DC, 1994, p. 518, ISBN 1-55581-048-9 Search PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- J. Neeser and T. Schweizer, Anal. Biochem., 1984, 142, 58–67 CrossRef CAS.
- F. François, C. Lombard, J. M. Guigner, P. Soreau, F. Brian-Jaisson, G. Martino, M. Vandervennet, D. Garcia, A. L. Molinier, D. Pignol, J. Peduzzi, S. Zirah and S. Rebuffata, Appl. Environ. Microbiol., 2012, 78, 1097–1106 CrossRef PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- M. O. Jaroszuk, M. Jaszek, M. M. Dudka, A. Blachowicz, T. P. Rejczak, G. Janusz, J. Wydrych, J. Polak, A. J. Wilkolazka and M. K. Szerszen, BioMed Res. Int., 2014 DOI:10.1155/2014/743812.
- B. Martin, W. Chadwick, T. Yi, S. S. Park, D. Lu, B. Ni, S. Gadkaree, K. Farhang, K. G. Becker and S. Maudsley, PLoS One, 2012, 7, e36911 CAS.
- D. Abd-El-Haleem, R. Al-Thani, T. Al-Mokemy, S. Al-Marii and F. Hassan, Pol. J. Microbiol., 2008, 57, 231–239 CAS.
- L. Peng, C. Yang, G. Zeng, L. Wang, C. Dai, Z. Long, H. Liu and Y. Zhong, Appl. Microbiol. Biotechnol., 2014, 98, 6847–6858 CrossRef CAS PubMed.
- T. C. Lin, J. S. Chang and C. C. Young, Biotechnol. Lett., 2008, 30, 1201–1206 CrossRef CAS PubMed.
- M. M. Bhat, S. Shankar, Y. M. Shikha and R. N. Shukla, Adv. Appl. Sci. Res., 2011, 2, 321–326 CAS.
- T. Gutierrez, D. Berry, T. Yang, S. Mishamandani, L. McKay, A. Teske and M. D. Aitken, PLoS One, 2013, 8, e67717 CAS.
- M. J. Coon, Biochem. Biophys. Res. Commun., 2005, 338, 378–385 CrossRef CAS PubMed.
- S. N. Singh, B. Kumari and S. Mishra, Environ. Sci. Eng., Springer-Verlag, Berlin, Heidelberg, 2012, DOI:10.1007/978-3-642-23789-8_17.
- E. Z. Gomaa, Pol. J. Microbiol., 2012, 61, 281–289 Search PubMed.
- S. B. Deng, R. B. Bai, X. M. Hu and Q. Luo, Appl. Microbiol. Biotechnol., 2003, 60, 588–593 CrossRef CAS PubMed.
- J. He, J. Zou, Z. Shao and J. Zhang, World J. Microbiol. Biotechnol., 2010, 26, 1135–1141 CrossRef CAS.
- I. W. Sutherland, Polysaccharides from microorganisms, plants and animals, ed. E. Vandamme, S. de Baets and A. Steinbuchel, Wiley–VCH Publisher, Weinheim, 2002, pp. 1–19 Search PubMed.
- D. J. Wozniak, T. J. O. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O’Toole and M. R. Parsek, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 7907–7912 CrossRef CAS PubMed.
- Q. Gao, U. Nilsson, L. L. Ilag and C. Leck, Anal. Bioanal. Chem., 2011, 399, 2517–2529 CrossRef CAS PubMed.
- H. Zhao, H. Liu and J. Zhou, Bioresour. Technol., 2013, 137, 226–232 CrossRef CAS PubMed.
- Z. Chunying, X. Aihua, W. Buyun, Y. Xianghui, H. Wentao, Y. Baokun, C. Changhong, L. Hongtao and Z. Jiangang, Colloids Surf., B, 2014, 122, 583–590 CrossRef PubMed.
- J. A. Morillo, M. Aguilera, A. R. Cormenzana and M. M. Sanchez, Curr. Microbiol., 2006, 53, 189–193 CrossRef CAS PubMed.
- S. Comte, G. Guibaud and M. Baudu, Process Biochem., 2006, 41, 815–823 CrossRef CAS PubMed.
- D. C. Sobeck and M. J. Higgins, Water Res., 2002, 36, 527–538 CrossRef CAS.
- J. Lin and C. Harichund, Afr. J. Biotechnol., 2012, 11, 9619–9629 CAS.
| This journal is © The Royal Society of Chemistry 2015 |