Synthesis and characterization of a chitosan/montmorillonite/ZrO2 nanocomposite and its application as an adsorbent for removal of fluoride
Abstract
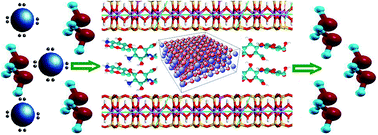
A series of chitosan/montmorillonlite/zirconium oxide (CTS/MMT/ZrO2) nanocomposites were made by varying the molar ratios of chitosan (CTS) to montmorillonite/zirconium oxide (MMT/ZrO2). The nanocomposites were characterized by FTIR, XRD and SEM. The surface area, pore volume and the pore size distribution of the CTS/MMT/ZrO2 were calculated by the BET technique. The effects of various molar ratios of CTS to MMT/ZrO2, the initial pH value of the fluoride solution, contact time, and temperature on adsorption capacities of samples for fluoride, adsorbent dose and initial concentration of fluoride were also investigated. The optimal conditions for removal of fluoride were found to be: molar ratio of CTS/MMT/ZrO2, 1 : 1; pH: 4; temperature: 30 °C for 60 min in 25.00 mL of 20 mg L−1 of fluoride solutions and 0.1 g of adsorbent. The fluoride adsorption capacity of CTS/MMT/ZrO2 was also found to be 23 mg g−1 experimentally. The adsorption capacities of CTS, MMT, ZrO2, CTS/ZrO2, CTS/MMT and CTS/MMT/ZrO2 nanocomposites for fluoride removal were compared. The results indicated that the adsorption capacity of the CTS/MMT/ZrO2 nanocomposite was higher than the average values of those of CTS (52 mg kg−1 for fluoride removal), MMT, ZrO2, CTS/ZrO2 and CTS/MMT. The adsorption kinetics and isotherms were also examined. It was found that all the sorption processes were better described by a pseudo-second-order equation and the Langmuir equation.

 Please wait while we load your content...
Please wait while we load your content...