Linear and three-arm star hydroxytelechelic poly(benzyl β-malolactonate)s: a straightforward one-step synthesis through ring-opening polymerization†
Abstract
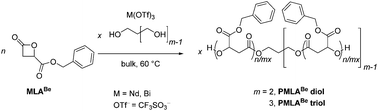
Ring-opening polymerization (ROP) of racemic-benzyl β-malolactonate (MLABe) initiated by an alcohol such as 1,3-propanediol (PPD) or 2-hydroxymethyl-1,3-propanediol (TMM), and catalyzed by a metal triflate M(OTf)3 with M = Nd, Bi, proceeded under mild operating conditions (in bulk at 60 °C). The functionality of the alcohol dictates the topology of the resulting hydroxy telechelic PMLABe. The ROP promoted by the neodymium-based catalytic system afforded a satisfactory activity and control in terms of molar mass and dispersity values (Mn,NMR up to 7000 g mol−1, ĐM < 1.35). Mechanistic insights revealed that ring-opening of MLABe took place through the selective oxygen–acyl bond cleavage without undesirable side reactions such as transesterification or crotonisation, as evidenced by NMR and mass spectrometry analyses of the recovered polyesters. The structure of the corresponding α,ω-hydroxy telechelic PMLABes was ascertained by 1H and 13C{1H} NMR, SEC, and MALDI-ToF mass spectrometry analyses. In comparison, methane and trifluoromethane sulfonic acids did not allow the formation of well-defined PMLABe diols. Differences in the behavior of MLABe and the related β-butyrolactone are highlighted. The present Nd(OTf)3/PPD or TMM catalytic ROP of MLABe thus represents a valuable direct synthesis of PMLABe diols and triols, respectively, without requiring chemical modification of a preformed PMLABe precursor.

 Please wait while we load your content...
Please wait while we load your content...