Simple structured polyetheramines, Jeffamines, as efficient cathode interfacial layers for organic photovoltaics providing power conversion efficiencies up to 9.1%†
Abstract
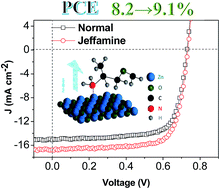
In this study, we have found that interfacial layers (IFLs) based on Jeffamines (industrial polyetheramine derivatives) can improve the performance of organic photovoltaics (OPVs). We evaluated four different Jeffamines (average molecular weight: 2000 g mol−1)—M2005, M2070, D2000, and ED2003—having either a monoamine or diamine structure and various ratios of propylene oxide (PO) and ethylene oxide (EO) for their suitability as IF materials for OPV applications. The presence of the Jeffamine altered the work function of ZnO and improved the electron transport, thereby causing the ZnO layers to function more efficiently as electron-selective electrodes. The power conversion efficiencies (PCEs) of inverted devices having the layered configuration glass/indium tin oxide (ITO)/ZnO (with or without the Jeffamine)/PTB7:PC71BM/MoO3/Ag increased from 8.1 ± 0.11 to 8.6 ± 0.37% when containing the Jeffamine-D2000 under illumination with AM 1.5G solar light (1000 W m−2), the result of a significantly increased fill factor (FF). The greatest OPV performance was that of the device incorporating Jeffamine-D2000—a PCE of 9.1% and a remarkable FF of 74.2%.
- This article is part of the themed collection: JMC A Editor’s choice collection: Recent advances in photovoltaics


 Please wait while we load your content...
Please wait while we load your content...