Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines†
Roberto
Adamo
*a,
Qi-Ying
Hu
b,
Antonella
Torosantucci
c,
Stefano
Crotti
a,
Giulia
Brogioni
a,
Martin
Allan
b,
Paola
Chiani
c,
Carla
Bromuro
c,
Douglas
Quinn
b,
Marta
Tontini
a and
Francesco
Berti
a
aNovartis Vaccines, Via Fiorentina 1, 53100 Siena, Italy. E-mail: roberto.adamo@novartis.com; Tel: +39-057-539393
bNovartis Institutes for BioMedical Research, 100 Technology Square, Cambridge, MA 02139, USA
cDepartment of Infectious, Parasitic and Immune-mediated Diseases, Istituto Superiore di Sanità, Viale Regina Elena, 299, 00161 Rome, Italy
First published on 3rd July 2014
Abstract
The elucidation of the molecular details underlying the immune properties of glycoconjugate vaccines has largely focused on the carbohydrate moiety, while very little is known on the effect of the corresponding conjugation sites. Herein we constructed a set of β-(1 → 3) glucan oligosaccharide conjugates with a well-defined glycan structure, connected to patterns of predetermined tyrosine or lysine residues onto the CRM197 carrier protein. To evaluate the effect of multivalent architecture in the glycan presentation, a novel linker enabling tyrosine-directed ligation of couples of oligosaccharides was prepared. The potential of these constructs as anti-Candida vaccines was evaluated in vivo, using as controls glycoconjugates prepared by a conventional random coupling strategy, and the structure–immune properties relationship was established. We found that: (i) the tyrosine-directed ligation resulted in higher anti-glycan IgG levels in comparison to the conjugation at predetermined lysine residues; (ii) the presentation of the carbohydrate antigen with a biantennary cluster of glycans onto specific tyrosine residues did not further increase the anti-glycan antibody level; (iii) the sera deriving from immunization with defined conjugates at tyrosine and, particularly at lysine residues, were proven stronger inhibitors of fungal adhesion to human epithelial cells in comparison to those from conjugates prepared by classic random chemistry; (iv) the presence of antibodies directed to the linkers did not affect the anti-glycan immune response. These findings suggest that a careful choice of the defined sites of conjugation and the loading density of antigens are important factors to raise high-quality anti-carbohydrate antibodies.
Introduction
Carbohydrates coating the surface of bacterial pathogens, have found application over the last few decades in the development of effective vaccines against many infectious diseases.1 Glycans are per se T-cell independent 2 (TI-2) antigens and, with the exception of certain zwitterionic polysaccharides, are unable to activate T cells and induce a memory response.2 However, the covalent linkage to protein carriers enable their uptake by antigen presenting cells (APCs).3 It is classically believed that following intracellular digestion of the protein portion by proteases, peptide epitopes generated from glycoconjugates are re-exposed on the cell surface in the context of Major Histocompatibility Complex class II (MHCII) and presented to CD4+ T-cells.3 Cytokines released by peptide/MHCII-activated T-cells can stimulate B-cell maturation to memory cells and trigger IgM to IgG class switching, with consequent induction of long-lasting protection even among infants and the elderly.3 Some early studies have evidenced that the carbohydrate portion of glycopeptides exposed in association to MHCII might be also critical for the T cell recognition.4 The involvement of the glycan from glycopeptides produced by endolysosomial digestion of glycoconjugates in the T cell recruitment has been recently corroborated by the isolation of sugar-specific CD4+ T-cell clones.5 The coexistence of different processing mechanisms for diverse glycoconjugates cannot be excluded, however in this model it can be speculated that different glycopeptides could be generated depending on the diverse residue to which the carbohydrate is connected, thus affecting the immunological properties of the glycoconjugate vaccines.6 The efficacy of the conjugate vaccines is determined by a series of interdependent variables, including the carbohydrate moiety, the loading of glycans incorporated onto the protein, and the linker used for conjugation.1,7 Importantly, when carbohydrates are covalently linked to other biomolecules such as proteins, avidity and selectivity of specific interactions are strongly dependent on the density and the spatial organization of the sugar moieties.8 Hence, the multivalent exposition of the glycans onto the carrier protein is another parameter that might influence both the carbohydrate–antigen presenting cells and the MHCII–CD4+ T-cell interaction.9 In most cases glycoconjugate vaccines are prepared from complex mixtures of heterogeneous bacterial polysaccharides covalently linked through non-specific methods to the carrier protein. After the introduction in 2004 of the first vaccine based on large-scale production of a synthetic oligosaccharide from H. influenzae type b,9 a lot of effort has been expended in the carbohydrate synthesis. Synthetic glycans, besides lacking bacterial impurities, present the advantage of having well-defined structures and bearing at their reducing end an arm suitable for chemoselective conjugation.10 By the use of synthetic carbohydrates, the effect on the immune response of glycan-related variables (length,11 non-end terminal residues12 or presence of charged functional groups13) have been explored. However, even when defined oligosaccharides are utilized, the conjugation is generally directed to the lysine residues of the carrier protein through highly random and hard to control methods.7 As a consequence, the impact of the conjugation site as well as the carbohydrate loading on the immune properties cannot be easily deciphered.Over the last few years new methods for site selective insertion of glycans either on non-natural or natural amino acidic residues are emerging,14 and an elegant method based on cysteine modification, leading to the preparation of glycotherapeutics uniform in glycan and attachment site modification, has been disclosed.15 In this example, however, capacity of the protein to function as a carrier for the sugar and study of the effect of glycosylated site on the immunogenicity of the glycoprotein were not detailed. Recently, we have addressed the use of 1,2,4-triazoledicarboxylate reagents to achieve tyrosine-directed conjugation of carbohydrate haptens to proteins.16 This approach allowed us to chemically manipulate the tyrosine residues of the cross reactive material of diphtheria toxin (CRM197),17 a carrier protein of primary importance for the manufacturing of many licensed vaccines,1,7 and insert sugar moieties into defined sites (Fig. 1A). For that study a β-(1→3) glucan hexamer was selected as the antigen,18 based on our previous finding that this short glycan fragment was able to induce murine IgG titres comparable to the structurally more complex Laminarin (Lam)–CRM197 conjugate, a well-established anti-Candida vaccine at the preclinical level.19,20 We have also demonstrated that a two-step conjugation procedure can be used in combination with the LC-MS/MS analysis of the proteolytic digests from linker-labelled carrier proteins to guide the conjugation of glycans to the more reactive lysine residues.21 By this strategy the β-(1→3) glucan hexasaccharide was conjugated at pre-determined lysines of CRM197. In both conjugation methods, the Cu(I) catalyzed Huisgen [3 + 2] cycloaddition between an azide and alkyne22 has been used for the condensation of glycan haptens to proteins modified with suitable linkers. This reaction offers chemoselectivity for targeting different protein functional groups and large flexibility for the introduction of a variety of carbohydrate moieties at pre-determined conjugation sites.16,21 The newly developed conjugation methods offer potent tools to create different patterns of carbohydrates with defined attachment points onto the carrier protein (Fig. 1), and unravel the effect of the resulting glycan loading/density on the immune response. Following our previous work, in this investigation we constructed β-(1 → 3) glucan oligosaccharide conjugates with well-defined glycan structure, either selectively coupled to tyrosine residues or connected to the more surface available lysine residues of the CRM197 carrier protein. A novel linker for tyrosine-directed ligation, enabling conjugation of clusters of two glycans to the carrier protein, was synthesized. By this approach the multivalent exposition of two sugar moieties attached to the same site was achieved. The anti-β-glucan antibody responses induced in vaccinated mice was thoroughly evaluated, in comparison to glycoconjugates prepared by a conventional random conjugation strategy. The polyclonal anti-β-glucan antibodies elicited by mice immunization were finally analysed for their capacity of recognizing the β-glucans expressed on the surface of C. albicans cells and to inhibit the fungal adhesion to human epithelial cells. In this manner the effect of the glycan incorporation with different patterns of defined conjugation sites was evaluated and the structure–immunogenicity relationship (SIR) was established. Since it has been reported that the use of Cu(I) catalyzed Huisgen azide–alkyne [3 + 2] cycloaddition can result in the induction of anti-linker antibodies which may decrease the immune response against the carbohydrate antigen,23 the structure–anti linker antibody level relationship was also assessed.
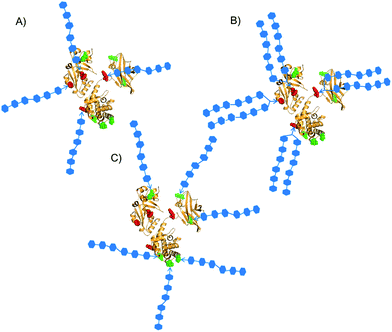 | ||
| Fig. 1 Patterns of defined sites for conjugation of synthetic glycans. Y27, Y46, Y358 and Y380 are red-highlighted; K103, K221, K236, K242, K498, K526 are green-highlighted.24 Three different types of constructs were designed: selective attachment of (A) oligomers or (B) bivalent clusters of oligomers on tyrosine residues; (C) attachment of oligomers on the more surface accessible lysine residues. | ||
Results and discussion
Preparation of the glycoconjugates
We have already developed procedures for the preparation of glycoconjugates with the defined β-(1→3) glucan hexasaccharide 7 linked to predetermined tyrosine16 or lysine21 residues of CRM197. To ascertain the capability of these well-defined conjugates to elicit an antibody response against standard (Lam) and fungal (C. albicans) β-glucan and determine the structure–immunogenicity relationship, we synthesized a novel set of biomolecules described in Scheme 1. The conjugate 1 was prepared by defined conjugation at Y27, Y46, Y358 and Y380, as previously reported.16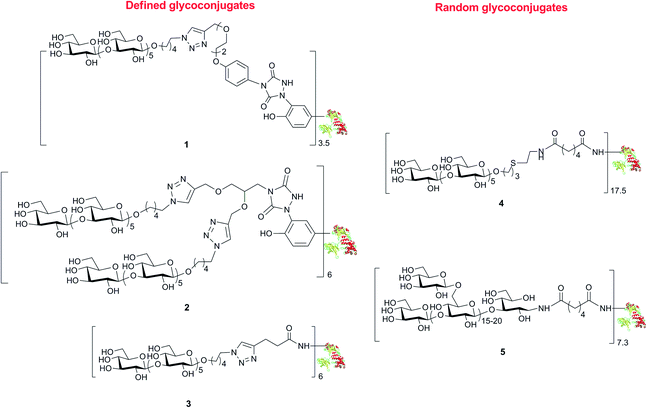 | ||
| Scheme 1 Structures of the set of glycoconjugates synthesized by random and defined conjugation methods. | ||
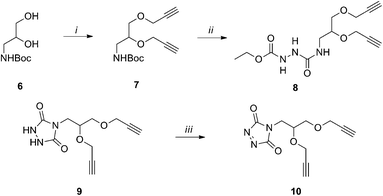
The binding of weak ligands such as sugars to proteins can be increased by effect of the multivalency.8,25 This principle has been recently applied to a series of carbohydrate-based clusters, dendrimers or nanoparticles, which have been proven able to interact with specific proteins more effectively than the glycan of origin.20 In the newly developed site directed ligation at tyrosine, we envisaged a flexible approach to achieve insertion of a cluster of two glycans at the same sites targeted for 1, and to explore the effect of the multivalent presentation of the carbohydrate antigens on the immunogenicity of the glycoconjugates. For this purpose, linker 10 was synthesized from amine 6 (Scheme 2), which was subjected to removal of the Boc protecting group and condensation with 4-nitrophenyl chloroformate, followed by addition of ethyl carbazate, to yield 8. After the base promoted formation of the triazolidine 9, oxidation by silica-supported nitric acid afforded the target molecule 10. The reaction of 10 with CRM197, under the previously described conditions,16 gave the modified protein 11 bearing two alkyne groups onto the same cluster ready for glycan coupling at defined tyrosine residues. The modified protein was purified by size exclusion chromatography and recovered at 82% yield, as determined according to its UV absorption at 210 nm. LC-MS analysis of the product showed an average incorporation of 3 bis-alkyne linkers (Scheme 3) (ESI†).
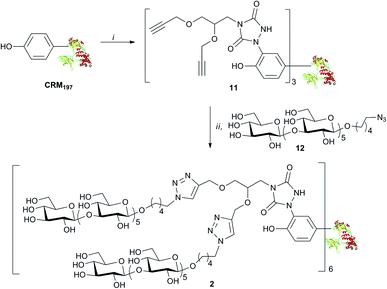 | ||
| Scheme 3 Preparation of the glycoconjugate 6: (i) 5, Tris–HCl, 82%; (ii) 7, Cu2SO4, Na ascorbate, 86%. | ||
MS/MS analysis of proteolytic digests determined that these linkers labelled primarily 4 out of the 18 tyrosine residues totally available, namely Y27, Y46, Y358 and Y380 (see ESI†).24 These modified sites correspond to the ones identified in conjugate 1.16 In accordance to our previous finding, we did not observe modification of other reactive amino acid residues including S, T, W, H, K, C and M, except W50 that was labelled only in trace amounts.16 Cu(I) catalyzed [3 + 2] cycloaddition of azide 12 with the bis-alkyne modified protein 11 gave 2, with a loading of 6 sugar moieties and a nearly quantitative condensation of the available alkyne functions. The lysine conjugate 3 bearing a limited number of carbohydrate moieties condensed at K103, K221, K236, K242, K498, K526, was synthesized as recently described.18 To obtain a glycoconjugate with defined carbohydrate structure but random distribution of the sugar hapten on 19 over the 40 available lysine residues of the carrier protein.21,26 conjugate 4 with a loading 17.5 hexamer moieties was assembled via active ester chemistry.18 Finally, the Lam–CRM197 conjugate 5, which has been demonstrated to induce protective anti-Candida antibodies, was prepared19 to be used as a benchmark in our immunological studies. This biomolecule presented a heterogeneous glycan structure and conjugated lysine residues.
Structure–immunogenicity relationship
In order to correlate the defined structure of 1–5 with their immune properties, we studied their proficiency to induce in vivo anti-Lam antibodies, which could cross-react with the β-glucan expressed on the surface of C. albicans and inhibit fungal adherence to human epithelial cells. With this intent, groups of 8 CD1 mice were immunized with three subcutaneous administrations of 2 μg (as carbohydrate content) of each glycoconjugate, two weeks apart. The formulations were adjuvanted with MF59,27 a water–oil emulsion used for anti-influenza vaccination, which has already been used in the preclinical testing of the Lam–CRM197 conjugate.20 Following the third immunization, mice sera were analysed by ELISA for their content of IgG recognizing Lam, a polysaccharide with a molecular structure similar to that of β-(1→3)-β-(1→6)-glucan onto the C. albicans surface. As expected, all the conjugates induced IgM antibodies at a statistically comparable level (Table 1). The switch IgM to IgG, that is indicative of the T-cell dependent memory response, was observed for all the tested glycoconjugates. The carbohydrate loading onto the protein is known as a key feature for the immunogenicity of the glycoconjugates. A number of examples, where defined synthetic oligosaccharide antigens were randomly bound to the lysine residues of the carrier protein, report that a lower glycosylation degree usually corresponds to lower specific IgG titers.28 In our case, no statistical difference was observed in the IgG levels induced by the diverse glycoconjugates. The conjugate at defined lysines 3 raised anti-β-glucan IgGs at a Geometric Mean Titer (GMT) similar to that exhibited by the Lam–CRM197 vaccine 5, whereas GMTs measured for the product of tyrosine-directed conjugation 1 and its counterpart 2 bearing clusters of two glycans onto the same tyrosine residues were three-fold and two-fold higher, respectively (Table 1). The conjugated hexasaccharide 4, with the highest level of glycosylation among the analysed biomolecules, induced IgG titers that were five-fold higher than both Lam–CRM1975, assembled by random conjugation chemistry, and the conjugate 3, prepared by well-defined lysine conjugation. Interestingly, the IgG levels were only two-fold higher than the 1 and 2 prepared by tyrosine-directed coupling. Overall the capability of the conjugates to generate anti-glucan antibodies followed the order: 4 > 1 ≈ 2 > 3 ≈ 5. Therefore, a trend was observed indicating that conjugation at tyrosine residues induced a higher IgG level, than conjugation at lysine residues. These results highlight that the attachment point of the glycan moiety is important for the efficacy of conjugate vaccines. Interestingly, the presentation of one or two glycans onto the same attachment site, as in conjugates 1 and 2, gave comparable results in terms of elicited IgG titers. This evidence suggests that this modification did not affect glycoconjugate uptake by the APCs or its exposure onto the MCHII to the T-cell.4,6| Anti-glucan antibody levels | ||||||
|---|---|---|---|---|---|---|
| Cmpound | Conjugation sites | Conjugate loading | IgM titer (GMT95% CI) | IgG titer (GMT95% CI) | IgG titer (GMT95% CI) with SCN 0.5 M | IgG without SCN/IgG with SCN |
| PBS | — | — | 153 (52–452) | 48 (28–84) | 2 | 24 |
| 1 | Y27, Y46, Y358 Y380 | 3.5 | 480(169–1361) | 1533 (395–5952) | 352 (71–1747) | 4 |
| 2 | Y27, Y46, Y358 Y380 | 6.0 | 912 (346–2404) | 1165 (234–5798) | 397 (65–2436) | 3 |
| 3 | K103, K221, K236, K242, K498, K526 | 6.0 | 327 (170–628) | 613 (112–3142) | 111 (16–758) | 6 |
| 4 | Undefined | 17.5 | 1100 (425–2850) | 2915 (785–10826) | 899 (158–5121) | 3 |
| 5 | Undefined | 7.3 | 1046 (418–2617) | 591 (161–2174) | 141 (42–472) | 4 |
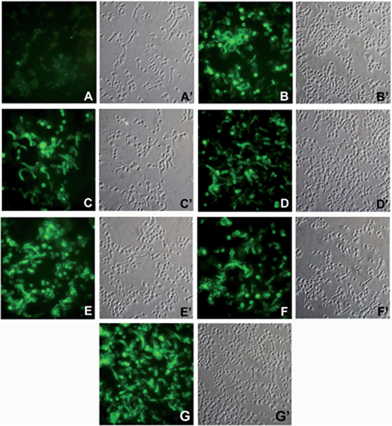
Anti β-glucan antibodies, either raised by active immunization with glucan-based vaccines or passively administered in the form of polyclonal or monoclonal antibody preparations, can induce significant protection, in animal models, against systemic and mucosal experimental infections with the human fungal pathogen C. albicans.19,29 An essential prerequisite for this protective activity is the capacity to recognize the β-(1→3)-β-(1→6) glucan that is present on the fungal cell wall, both as a structural component of the rigid wall skeleton and as the glycan moiety of proteins, often virulence-related, located in the outer wall layer.29 Immunofluorescence staining experiments, as shown in Fig. 2, indicated that our glycoconjugates 1–5 were all similarly able to induce antibodies binding the surface of C. albicans cells.29,30 The pattern of binding included both yeast cells and hyphal filaments, and closely resembled what was previously observed using polyclonal or monoclonal anti-β-glucan antibody preparations endowed with proven protective activity in vivo.19,29
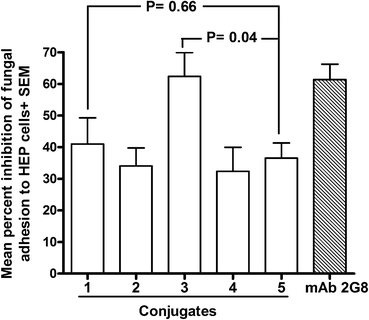
We have demonstrated that a critical, protection-related biological activity of anti-β-glucan antibodies is the ability to interfere with important virulence properties of C. albicans such as adherence to host cells, a process which plays a key role in fungal invasion.29 Thus, we tested the sera obtained by immunization with the different conjugates for their capacity to inhibit the adhesion of C. albicans cells to cultured human epithelial (HEP-2), in comparison with a highly protective, anti β-glucan murine monoclonal antibody (mAb 2G831). All sera were found able to inhibit, to some extent, the adhesion process (range: 32.3–62.3% as compared to cultures with an irrelevant (PBS) control serum; Fig. 3). Importantly, however, the sera obtained with the conjugates 1 and 3, which were prepared by defined conjugation at the lysine and tyrosine residues, respectively, resulted in the strongest inhibitors. The reduction of fungal adherence induced by these sera was higher than that provoked by those obtained by immunization with Lam–CRM1975, a glycoconjugate which has proven protective in vivo.29 In particular, the serum from mice immunized with the conjugate 3 exhibited an inhibition value (62.3%) which was significantly higher than that measured for the Lam–CRM1975 serum (p < 0.05), and totally comparable to that measured for the anti-C. albicans protective mAb 2G8. It is also noteworthy that the ability to inhibit fungal adherence was not correlated to the levels of Lam-reacting antibodies measured for the different sera (see Fig. 2). This would suggest that, among the repertoire of anti β-glucan antibodies raised by the different conjugates, only some antibodies are efficacious in contrasting fungal adhesion and that these anti-adhesive antibodies can be elicited with different efficacy by the different conjugates. Again, sera from conjugate 2, where clusters of two glycans has been installed onto the same conjugation sites of 1, exhibited an inhibitory activity comparable to 4 and 5, but lower than 3, indicating that no benefit could be achieved by the multivalent architecture of this glycoconjugate.
Notably, compound 4, prepared by random conjugation of the synthetic hexamer, showed an inhibitory activity comparable to that of 2 and 5.
To summarize, the conjugates induced sera with a potency as inhibitors of fungal adherence in the order: 3 > 1 > 2 ≈ 4 ≈ 5.
It is worth mentioning that Cu(I) salts are known to be cytotoxic. This has prevented the use of Cu(I) for imaging studies in living organisms.31 In the present investigation the glycoconjugates employed for administration in mice were purified by dialysis. Although we did not estimate the residual Cu(I) content in the samples, the amount used in the glycan condensation would be in the order of pg per dose of vaccine, far below the proposed permitted daily exposure for parental route of 5 μg Cu per kg per day.32
Structure–anti linker antibodies relationship
It is known that rigid and constrained spacers might elicit undesirable antibodies potentially diverting the immune response from the carbohydrate hapten.1 Therefore, the murine sera obtained from vaccination with 1–5 would contain mixtures of antibodies directed to the glycan, the spacer and the protein epitopes. We took advantage of the synthesized set of conjugates with a defined attachment site to investigate the correlation of the anti-linker response and the neo-epitopes generated by the attachment of the linker. To determine exclusively the level of antibodies against the linker, while ruling out possible interference from those against the glycans and the protein, we assayed the polyclonal sera for reactivity towards a series of constructs composed of the same linkers employed in our tested biomolecules but coupled, at tyrosine or lysine conjugation sites, to non-CRM197 proteins (Scheme 4, see ESI† for details). The serum generated from each of the conjugates 1–4 was tested against each of the constructs 13–16. By doing so, we could obtain information on the common portions, such as the triazole ring and the aliphatic chains, presented in the different linkers. Generally the level of IgGs measured against the ring containing linkers seemed to be higher than those against the carbohydrate, but it should be noticed that these antibody titers cannot be directly compared, because two substantially different coating reagents were used (the sole laminarin and the protein-based constructs 13–15, respectively). While we did find negligible antibody titers against the succinic spacer used for the active ester mediated conjugation in 4 (see ESI†), which is typically considered immunosilent, all the triazole containing linkers induced antibodies against their corresponding structures (Table 2). The conjugate inducing higher anti-linker antibodies was 1, which presented a phenyl ring in addition to the triazole generated by the click chemistry in conjunction with the aromatic ring of the tyrosine residue, followed by 2, where two triazole rings have been generated in concomitance to the tyrosine aromatic ring, and finally 3, which presented only the triazole system. When sera from conjugate 1 were tested against 14, where the triazole was the sole common portion in the structure, the measured antibody level was considerably lower than that found with 15 as coating reagent. Therefore, we conclude that anti-linker antibodies were primarily directed to the phenyl ring rather than to the triazole. To further investigate the nature of the antibodies directed to the carbohydrates and to the linkers generated by our glycoconjugates, we performed a modified ELISA analysis in the presence of 0.5 M ammonium thiocyanate (NH4SCN), a chaotropic salt able to dissociate antibody–antigen complexes of low avidity (Table 1 and ESI†).33 Importantly, levels of measured anti Lam antibodies were only partially affected when ELISA was carried out with NH4SCN, indicating that anti-carbohydrate antibodies with high avidity had been raised by the glycoconjugate vaccinations. Conversely, a remarkable decrease of the anti-linker antibody levels against the molecules 16 and 14 was measured in sera from 2- and 3-immunized mice in the presence of the chaotropic salt (p 0.03 and 0.003, respectively), while a two-fold reduction of the titers was observed when sera induced by conjugate 1 were analysed against reagent 15. These findings confirmed that linkers presenting the phenyl ring were more prone to induce anti-spacer antibodies at higher extent and with higher avidity, whereas when only the triazole was present in the linker, antibodies with lower avidity were mainly generated. The proficiency at inducing anti-spacer antibodies followed the order: 1 > 2 > 3. Importantly, with all the linkers, we did not observe any significant shift of the antibody response from the glycan antigens to the spacer.23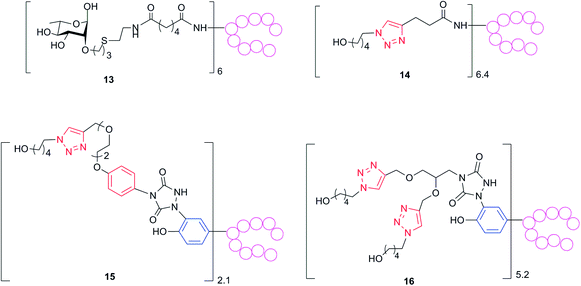 | ||
| Scheme 4 To measure anti-linker antibody levels, polyclonal sera from mice immunized with conjugates 1–5 were assayed against ELISA plates coated with reagents 13–16 prepared from non-CRM197 proteins (see ESI†). Red rings are inserted during the glycan conjugation. Blue rings belong to tyrosine residues. | ||
| Coating reagent | Construct 14 | Construct 15 | Construct 16 | ||||
|---|---|---|---|---|---|---|---|
| Anti-conjugate serum | IgG titer (GMT95% CI) | IgG titer (GMT95% CI) with SCN 0.5 M | IgG titer (GMT95% CI) | IgG titer (GMT95% CI) with SCN 0.5 M | IgG titer (GMT95% CI) | IgG titer (GMT95% CI) with SCN 0.5 M | IgG without SCN/IgG with SCN |
| 1 | 116 (23–587) | — | 34670 (18838–63808) | 20920 (9587–45651) | 290 (38–2191) | — | 2 |
| 2 | 62 (16–249) | — | 8 (1–84) | — | 16132 (8249–31548) | 1532 (273–8603) | 10 |
| 3 | 2090 (725–6026) | 41 (5–346) | 10 (2–59) | — | 164 (21–1252) | — | 51 |
| 4 | 78 (13–480.5) | — | 2 | — | 4 (1–17) | — | 21 |
Conclusions
Carbohydrate-based vaccines play a leading role in the prevention of life threatening infectious diseases caused by virulent pathogens. With progress in the assembly of complex oligosaccharides, it has been thoroughly explored how the sugar component influences the immune properties of these vaccines, independently from the corresponding linker and attachment points. We have recently developed methods for the installation of patterns of glycans onto defined attachment points of the carrier protein CRM197. By these approaches the effect of these conjugation site and the carbohydrate loading/density on the immune response can now be more systematically investigated.Using a synthetic β-glucan hexasaccharide as a model antigenic structure, we applied the tyrosine-directed ligation chemistry previously reported16 for defining the linker and the sites of conjugation on the carrier protein CRM197 of conjugate 1. Glycoconjugate 2 containing on the same conjugation sites multiple copies of a bivalent construct of the β-glucan hexamer was prepared for comparison. Additional constructs with the sugar hapten either connected at the more surface accessible lysine residues (3) or randomly linked to a larger number of lysine residues (4) were synthesised. As control, the glucan–CRM197 conjugate 5 previously shown to be highly protective against systemic and mucosal C. albicans infections in animal models was used.16,17 After immunization studies in mice, the antibody responses and inhibition potency of the anti-carbohydrate sera against fungal adhesion to human epithelial cells were analysed and compared. We found that the synthetic glycoconjugates with a limited number of a relatively short synthetic hexasaccharide at the defined tyrosine or lysine sites on CRM197 elicited quality anti-fungal β-glucan IgG titers comparable to those raised by the Lam–CRM197 conjugate 5 that contains much longer natural fungal β-glucans through random lysine conjugation. In particular, the anti-sera of the defined synthetic glycoconjugate 3, showed more potent inhibition activity against host cell adherence than that of the Lam–CRM197 vaccine 5. The installation of a bivalent cluster of glycans onto specific tyrosine residues further increased neither the anti-carbohydrate antibody response nor the functionality of the sera against the fungal adhesion in the present case. Even if relatively strong antibody responses against the aromatic linker (present in the tyrosine-directed ligation chemistry) or moderate antibody response against the triazole linker (resulting from the alkyne–azide Cu(I) catalyzed [3 + 2] cycloaddition) were elicited, this did not appear to significantly affect the quality of the anti-glycan antibodies. Our results suggest that a careful choice of the defined sites of conjugation, the size of the glucan antigens, and probably the loading density of antigens are important factors to raise high-quality IgG antibodies to block fungal adhesion to host epithelial cells.
We expect that the use of defined constructs will aid a better understanding of the parameters which influence the activity of glycoconjugate vaccines and open new perspectives in the study and selection of candidate molecules.
Experimental
Procedure for CRM197 labeling16
To a solution of CRM197 (2.92 mg, 0.05 μmol) in Tris–HCl (2.0 mL, 0.2 M, pH 7.4) was added 10 additions of freshly prepared reagent 10 (2.5 μL, 100 mM in CH3CN, 0.25 μmol) every minute (50 eq. in total). The mixture was agitated at 20 °C for 30 min. The mixture was passed through a Zeba 7K MWCO spin column with PBS (pH 7.4) as eluting buffer three times. The bisalkyne–CRM19711 (2 mL, 1.2 mg mL−1) was obtained at 82% yield (the recovery was determined according to the UV absorption at 210 nm in comparison with the standard). An average incorporation of 3 bis-alkyne linkers was determined by LC-ESI MS (see ESI†). These linkers were inserted onto 4 highly modified tyrosine residues (Y27, Y46, Y358 and Y380) per protein, as estimated by comparison of the peak intensity of the ESI-MS spectra of peptides derived from protein digestion (see ESI†).Procedure for glycosylation by copper catalyzed click chemistry16
To a solution of 6 (300 μg, 0.005 μmol) in 100 mM sodium phosphate pH 7.0 (70 μL) and 7 (0.10 μmol), a premixed solution of 5 mM CuSO4·5H2O (5 μL) and 25 mM THPTA (5 μL) was added under a nitrogen atmosphere, followed by 5 mM aminoguanidine hydrochloride (5 μL) and 10 mM sodium ascorbate (5 μL). After gently stirring for 1.5 h at room temperature, the glycoconjugate was washed on a 30 kDa Amicon centrifugal filter with 10 mM EDTA/10 mM sodium phosphate pH 7.0 (2 × 100 μL) and 10 mM sodium phosphate pH 7.0 (8 × 100 μL), and subsequently reconstituted with 10 mM sodium phosphate (pH 7.0). Yield (recovered 2, based on micro BCA, Pierce, determination of protein content): 85%. The level of carbohydrate incorporation was assessed by MALDI-TOF MS (UltraFlex III MALDI-TOF/TOF, Bruker Daltonics) in linear mode and with positive ion detection. The samples for analysis were desalted and prepared by mixing 2.5 μL of product and 2.5 μL of Super DHB or sinapinic acid matrix; 2.5 μL of each mixture was deposited on a samples plate, dried at room temperature for 10 min, and subjected to the spectrometer. A carbohydrate loading of 6 moieties was found. The yield of azide–alkyne condensation was 86%.Mice immunization
Animal experimental guidelines set forth by the Novartis Animal Care Department were followed in all animal studies performed. Groups of eight female CD1 mice were immunized at days 1, 14, and 28 with glycoconjugate 1–5 containing 2 μg of carbohydrate antigens (Laminarin or glucan hexasaccharide) or negative control (PBS). The carbohydrate content of samples 1–4 was calculated as follows.11,18 First the protein concentration was determined by microBCA (Pierce). The carbohydrate–protein ratio was then assessed by MALDI TOF MS analysis, as described above. Based on this ratio the amount of sugar in the samples containing conjugates 1–4 was calculated. Laminarin content in conjugate 5 was determined by HPAED-PAD analysis using a CarboPac PA1 column (50 mm × 250 mm) coupled to a CarboPac PA1 guard column and connected to a Dionex ICS3000 system, as previously reported.20 The glycoconjugates were formulated with MF59 and delivered in a volume of 150 μL by subcutaneous injection. Bleedings were performed at days 0, 27, and 42.ELISA analysis
Ninety-six-well Maxisorp plates (Nunc, Thermo Fisher Scientific) were coated overnight at 4 °C with laminarin 5 μg per well in 0.05 M bicarbonate buffer at pH 9.6. After coating, the plates were washed three times with 300 μL per well of TPBS (PBS with 0.05% Tween 20, pH 7.4) and blocked with 100 μL per well of 3% BSA (Sigma-Aldrich) for 1 h at 37 °C. Subsequently, each incubation step was followed by a triple TPBS wash. Sera, prediluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25–1
25–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in TPBS, were transferred into coated-plates (200 μL) and then serially two-fold diluted. After 2 h incubation at 37 °C, 100 μL per well of 1
100 in TPBS, were transferred into coated-plates (200 μL) and then serially two-fold diluted. After 2 h incubation at 37 °C, 100 μL per well of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in TPBS antimouse IgM or IgG alkaline phosphatase conjugated (Sigma-Aldrich) were added, and plates were incubated for 1 h at 37 °C. Plates were then developed for 30 min at room temperature with 100 μL per well of 1 mg mL−1p-nitrophenyl phosphate disodium (Sigma-Aldrich) in 1 M diethanolamine (pH 9.8) and read at 405 nm with a microplate spectrophotometer (Biorad). Antibody titres were defined as reciprocal of sera dilution using an optical density (OD) of 1. The statistical and graphical analysis was performed using GraphPad Prism 5 software by applying the Mann Withney as statistical analysis. For ELISA HA, sera were pre-diluted with 0.5 M NH4SCN and used as described above.33
000 in TPBS antimouse IgM or IgG alkaline phosphatase conjugated (Sigma-Aldrich) were added, and plates were incubated for 1 h at 37 °C. Plates were then developed for 30 min at room temperature with 100 μL per well of 1 mg mL−1p-nitrophenyl phosphate disodium (Sigma-Aldrich) in 1 M diethanolamine (pH 9.8) and read at 405 nm with a microplate spectrophotometer (Biorad). Antibody titres were defined as reciprocal of sera dilution using an optical density (OD) of 1. The statistical and graphical analysis was performed using GraphPad Prism 5 software by applying the Mann Withney as statistical analysis. For ELISA HA, sera were pre-diluted with 0.5 M NH4SCN and used as described above.33
Microorganism
The C. albicans strains BP (type collection of the Istituto Superiore di Sanità) was used throughout the study. Yeast cells, from stock cultures in Sabouraud-dextrose agar (Difco-BBL, Franklin Lakes, New York), were grown in Winge medium at 28 °C for 24 h, then harvested by centrifugation, washed, counted in a hemocytometer, and resuspended to the desired concentration in phosphate-buffered saline (PBS). Fungal germ-tubes were obtained by culturing yeast cells at 37 °C in Lee's medium, as previously described.34Murine monoclonal antibody (mAb) 2G8
The mAb 2G8 was generated in mice previously immunized with a solubilized preparation of C. albicans cell wall β-glucan conjugated to CRM197.29 MAb isotype, specificity, biological activities and protective efficacy in vivo against C. albicans infections has been previously reported in detail.29 The mAb was precipitated from culture supernatants of the producing hybridoma by ammonium sulfate, dialysed against PBS, purified by affinity onto protein A Sepharose 4B (GE Healthcare, Fairfield, USA) and quantified by a commercial protein assay (BioRad, Richmond, USA). An irrelevant, mAb of the same isotype was prepared following an analogous procedure to serve as the negative control.Immunofluorescence microscopy
For immunofluorescence staining, C. albicans germ-tubes were allowed to adhere on immunofluorescence microscope slides, extensively washed with PBS containing 0.1% Tween 20 and blocked (1 h, 37 °C) with 3% bovine serum albumin (BSA) in PBS. Spots were reacted (2 h, 37 °C) with various dilutions of the sera in PBS-3% BSA, washed again, and treated with fluorescein isothiocyanate-(FITC)-conjugated-anti mouse IgG (Sigma-Aldrich, 1 h, 37 °C). After extensive washings, the slides were mounted in glycerol, pH 9.6, and examined under a Leitz Diaplan fluorescence microscope.Adhesion assays
Hep-2 cells monolayers (1.5 × 105 cells per well) were washed with PBS and incubated with Candida cells (4 × 104 per well) in PBS containing immune or control sera at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution or protein A affinity-purified mAb 2G8 or irrelevant, control mAb at the dose of 150 μg mL−1. All conditions were assayed in triplicate. After incubation for 2 h at 37 °C, non-adherent fungal cells were removed with repeated, gentle washings with PBS. Adherent fungi were recovered from cell monolayers with PBS containing 0.2% Triton X-100 and enumerated by standard CFU counts. Percent inhibition of fungal adhesion was calculated by comparing the number of adherent fungi in wells containing the immune sera or the mAb 2G8 with that in wells containing equal concentrations of non-immune (PBS) serum or the irrelevant mAb, respectively.
2 dilution or protein A affinity-purified mAb 2G8 or irrelevant, control mAb at the dose of 150 μg mL−1. All conditions were assayed in triplicate. After incubation for 2 h at 37 °C, non-adherent fungal cells were removed with repeated, gentle washings with PBS. Adherent fungi were recovered from cell monolayers with PBS containing 0.2% Triton X-100 and enumerated by standard CFU counts. Percent inhibition of fungal adhesion was calculated by comparing the number of adherent fungi in wells containing the immune sera or the mAb 2G8 with that in wells containing equal concentrations of non-immune (PBS) serum or the irrelevant mAb, respectively.
Acknowledgements
We acknowledge Maria Rosaria Romano for providing the Lam–CRM197 conjugate.Notes and references
- P. Costantino, R. Rappuoli and F. Berti, Expert Opin. Drug Discovery, 2011, 6, 1045 CrossRef CAS PubMed.
- E. T. Clarke, N. A. Williams, J. Findlow, R. Borrow, R. S. Heyderman and A. Finn, J. Immunol., 2013, 191, 6071 CrossRef CAS PubMed.
- F. Y. Avci and D. L. Kasper, Annu. Rev. Immunol., 2010, 28, 107 CrossRef CAS PubMed.
- G. Y. Ishioka, A. G. Lamont, D. Thomson, N. Bulbow, F. C. Gaeta, A. Sette and H. M. Grey, J. Immunol., 1992, 148, 2446 CAS.
- F. Y. Avci, X. Li, M. Tsuji and D. L. Kasper, Nat. Med., 2011, 17, 1602 CrossRef CAS PubMed.
- F. Berti and R. Adamo, ACS Chem. Biol., 2013, 8, 1653 CrossRef CAS PubMed.
- R. Adamo, A. Nilo, B. Castagner, O. Boutureira, F. Berti and G. J. L. Bernardes, Chem. Sci., 2013, 4, 2995 RSC.
- A. Bernardi, J. Jiménez-Barbero, A. Casnati, C. De Castro, T. Darbre, F. Fieschi, J. Finne, H. Funken, K.-E. Jaeger, M. Lahmann, T. K. Lindhorst, M. Marradi, P. Messner, A. Molinaro, P. V. Murphy, C. Nativi, S. Oscarson, S. Penadés, F. Peri, R. J. Pieters, O. Renaudet, J.-L. Reymond, B. Richichi, J. Rojo, F. Sansone, C. Schaffer, B. W. Turnbull, T. Velasco-Torrijos, S. Vidal, S. Vincent, T. Wennekes, H. Zuilhofxy and A. Imberty, Chem. Soc. Rev., 2013, 42, 4709 RSC.
- V. Verez-Bencomo, V. Fernandez-Santana, E. Hardy, M. E. Toledo, M. C. Rodrıguez, L. Heynngnezz, A. Rodriguez, A. Baly, L. Herrera, M. Izquierdo, A. Villar, Y. Valdes, K. Cosme, M. L. Deler, M. Montane, E. Garcia, A. Ramos, A. Aguilar, E. Medina, G. Torano, I. Sosa, I. Hernandez, R. Martınez, A. Muzachio, A. Carmenates, L. Costa, F. Cardoso, C. Campa, M. Diaz and R. Roy, Science, 2004, 305, 522 CrossRef CAS PubMed.
- (a) V. Pozsgay, Curr. Top. Med. Chem., 2008, 8, 126 CrossRef CAS; (b) L. Morelli, L. Poletti and L. Lay, Eur. J. Org. Chem., 2011, 5723 CrossRef CAS; (c) C. Anish, B. Schumann, C. Lebev Pereira and P. H. Seeberger, Chem. Biol., 2014, 21, 38 CrossRef CAS PubMed.
- D. Safari, H. A. T. Dekker, J. A. F. Joosten, D. Michalik, A. C. d. Souza, R. Adamo, M. Lahmann, A. Sundgren, S. Oscarson, J. P. Kamerling and H. Snippe, Infect. Immun., 2008, 76, 4615 CrossRef CAS PubMed.
- B. Benaissa-Throuw, D. J. Lefeber, J. P. Kamerling, J. P. Vliegenthart, K. Kaaijeveld and H. Snippe, Infect. Immun., 2001, 69, 4968 Search PubMed.
- (a) A. V. Nikolaev, I. V. Botvinko and A. J. Ross, Carbohydr. Res., 2007, 342, 297 CrossRef CAS PubMed; (b) R. Adamo, M. R. Romano, F. Berti, R. Leuzzi, M. Tontini, E. Danieli, E. Cappelletti, O. S. Cakici, E. Swennen, V. Pinto, B. Brogioni, D. Proietti, C. L. Galeotti, L. Lay, M. A. Monteiro, M. Scarselli and P. Costantino, ACS Chem. Biol., 2012, 7, 1420 CrossRef CAS PubMed.
- (a) S. I. van Kasteren, H. B. Kramer, H. H. Jensen, S. J. Campbell, J. Kirkpatrick, N. J. Oldham, D. C. Anthony and B. G. Davis, Nature, 2007, 446, 1105 CrossRef CAS PubMed; (b) A. J. de Graaf, M. Kooijman, W. E. Hennink and E. Mastrobattista, Bioconjugate Chem., 2009, 20, 1281 CrossRef CAS PubMed; (c) G. J. L. Bernardes, E. J. Grayson, J. C. Errey and B. G. Davis, J. Am. Chem. Soc., 2008, 130, 5052 CrossRef CAS PubMed.
- E. J. Grayson, G. J. L. Bernardes, J. M. Chalker, O. J. Boutureira, R. Koeppe and B. G. Davis, Angew. Chem., Int. Ed., 2011, 50, 4127 CrossRef CAS PubMed.
- (a) Q.-Y. Hu, M. Allan, R. Adamo, D. Quinn, H. Zhai, G. Wu, K. Clark, J. Zhou, S. Ortiz, B. Wang, E. Danieli, S. Crotti, M. Tontini, G. Brogioni and F. Berti, Chem. Sci., 2013, 4, 3827 RSC; (b) R. Adamo, M. Allan, F. Berti, E. Danieli and Q.-Y. Hu, PCT Int. Appl., WO 2013009564A1, 2013.
- G. Giannini, R. Rappuoli and G. Ratti, Nucleic Acids Res., 1984, 12, 4063 CrossRef CAS PubMed.
- R. Adamo, M. Tontini, G. Brogioni, M. R. Romano, G. Costantini, E. Danieli, D. Proietti, F. Berti and P. Costantino, J. Carbohydr. Chem., 2011, 30, 249 CrossRef CAS.
- A. Torosantucci, C. Bromuro, P. Chiani, F. De Bernardis, F. Berti, C. Galli, F. Norelli, C. Bellucci, L. Polonelli, P. Costantino, R. Rappuoli and A. Cassone, J. Exp. Med., 2005, 202, 597 CrossRef CAS PubMed.
- C. Bromuro, M. Romano, P. Chiani, F. Berti, M. Tontini, D. Proietti, E. Mori, A. Torosantucci, P. Costantino, R. Rappuoli and A. Cassone, Vaccine, 2010, 28, 2615 CrossRef CAS PubMed.
- S. Crotti, H. Zhai, J. Zhou, M. Allan, D. Proietti, W. Pansegrau, Q.-Y. Hu, F. Berti and R. Adamo, ChemBioChem, 2014, 15, 836 CrossRef CAS PubMed.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tørnoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed.
- Z. Yin, H. G. Nguyen, S. Chowdhury, P. Bentley, M. A. Bruckman, A. Miermont, J. C. Gildersleeve, Q. Wang and X. Huang, Bioconjugate Chem., 2012, 23, 1694 CrossRef CAS PubMed.
- E. Malito, B. Bursulaya, C. Chen, P. Lo Surdo, M. Picchianti, E. Balducci, M. Biancucci, A. Brock, F. Berti, M. J. Bottomley, M. Nissum, P. Costantino, R. Rappuoli and G. Spraggon, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5229 CrossRef CAS PubMed.
- P. I. Kitov and D. R. Bundle, J. Am. Chem. Soc., 2003, 125, 16271 CrossRef CAS PubMed.
- A. Bardotti, G. Averani, F. Berti, S. Berti, V. Carinci, S. D'Ascenzi, B. Fabbri, S. Giannini, A. Giannozzi, C. Magagnoli, D. Proietti, F. Norelli, R. Rappuoli, S. Ricci and P. Costantino, Vaccine, 2008, 26, 2284 CrossRef CAS PubMed.
- D. T. O'Hagan, R. Rappuoli, E. De Gregorio, T. Tsai and G. Del Giudice, Expert Rev. Vaccines, 2011, 10, 447 CrossRef PubMed.
- (a) V. Poszgay, C. Chu, L. Pannell, J. Wolfe, J. B. Robbins and R. Schneerson, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5194 CrossRef; (b) F. Mawas, J. Niggemann, C. Jones, M. J. Corbel, J. P. Kamerling and J. F. G. Vliegenthart, Infect. Immun., 2002, 70, 5107 CrossRef CAS.
- A. Torosantucci, P. Chiani, C. Bromuro, F. De Bernardis, A. S. Palma, Y. Liu, G. Mignogna, B. Maras, M. Colone, A. Stringaro, S. Zamboni, T. Feizi and A. Cassone, PLoS One, 2009, 4, e5392 Search PubMed.
- E. Paulovicova, L. Paulovicova, R. Pilisiov, S. Bystricky, D. V. Yashunsky, A. A. Karelin, Y. E. Tsvetkov and N. E. Nifantiev, FEMS Yeast Res., 2013, 13, 659 CrossRef CAS PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793 CrossRef CAS PubMed.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_%20guideline/2009/09/WC500003587.pdf .
- D. M. Granoff, S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin and H. V. Raff, Clin. Diagn. Lab. Immunol., 1998, 5, 479 CAS.
- R. Romagnoli, G. Nisini, P. Chiani, S. Mariotti, R. Teloni, A. Cassone and A. Torosantucci, J. Leukocyte Biol., 2003, 75, 117 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra and ESI MS/MALDI TOF MS spectra. See DOI: 10.1039/c4sc01361a |
| This journal is © The Royal Society of Chemistry 2014 |