On the dynamics of kefir volatome†
Jie-Bi Hua,
Sampath Gunathilake‡
a,
Yu-Chie Chenab and
Pawel L. Urban*ab
aDepartment of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan. E-mail: plurban@nctu.edu.tw; Fax: +886-3-5723764
bInstitute of Molecular Science, National Chiao Tung University, Hsinchu, Taiwan
First published on 10th June 2014
Abstract
Probiotic drinks constitute an important component of the daily diet in the modern society. They contain active microbial strains, which contribute to the chemical composition of these dairy products. The metabolic activity of microbes can affect the flavor and smell of probiotic drinks at room temperature. “Kefir” is an example of fermented milk, which contains various species of bacteria as well as fungi (yeast). In order to gain a better understanding of the metabolic processes shaping the chemical composition of kefir drinks, in the present study, we perform a comparative analysis of volatomes of kefir and single-species cultures of Saccharomyces cerevisiae. Gas chromatography coupled with mass spectrometry enabled the monitoring of volatile metabolites collected onto solid phase microextraction fibres (divenylbenzene/carboxen/polydimethylsiloxane). The temporal profiles of secondary metabolites (isopentyl acetate, ethyl hexanoate, ethyl octanoate, phenethyl acetate, and ethyl decanoate), present in S. cerevisiae cultures and commercial kefir, exhibit similarity. This points to a significant contribution of Saccharomyces strains on the dynamics of the chemical composition of kefir during storage at ambient conditions. On the other hand, commercial yogurt, which contains various species of bacteria but not yeast is much more chemically stable and does not exhibit rapid changes in the ester-type metabolites, which are typical of kefir.
1. Introduction
The increasing awareness of a healthy lifestyle has led to an increased production and consumption of probiotic drinks. A daily intake of yogurt and other yogurt-like dairy products is believed to enrich gut microflora with live microorganisms that are beneficial to the host organism.1 For example, the microorganisms present in yogurt can stop the growth of harmful bacteria in the intestine,2 decrease levels of serum cholesterol,3 prevent gastrointestinal conditions (e.g. lactose intolerance, colon cancer, Helicobacter pylori infection, and others) as well as allergies.4 Yogurt is produced by the incubation of milk or cream with live and active bacterial cultures (Bifidobacterium sp., Lactobacillus sp., and Streptococcus sp.) but not yeast.5 During the production of yogurt, fermentative processes convert sugars to acids, alcohols as well as gaseous byproducts.6 Apart from yogurt, another probiotic drink, called “kefir”, has gained popularity across continents. Kefir contains yeast cells (Saccharomyces sp.) along with numerous species of bacteria living in a symbiosis manner.2,7,8 It is a foamy effervescent dairy product with a sour and alcoholic taste. Kefir is prepared by adding “kefir grains”, which contain a mixture of microbes, to fresh milk in order to start fermentation.2 The resulting kefir curd can be considered a biological (or ecological) entity on its own merits because it is more complex than all its microbial components, and it has unique biochemical properties. Importantly, when preparation of kefir is complete, the microorganisms are still alive and capable of metabolism.Due to the presence of active microbial strains in probiotic drinks, when probiotic products such as kefir, are removed from refrigeration, their chemical composition may change over the course of hours as the bacteria and yeast cells metabolise carbon-rich matrix components, releasing their metabolic byproducts. The release of metabolites by microorganisms found in probiotic drinks during production or storage is very important from the commercial point of view because surplus amounts of volatile compounds directly influence flavor, organoleptic properties, consumer acceptability, and shelf life.6,9 Concentrations of the metabolites produced by microorganisms present in probiotic drinks may vary over time. Due to the complex chemical and biological composition, difficulty in isolating and identifying individual species of microorganisms, and the symbiotic relationships, which may act on cellular metabolism, it is not straightforward to monitor the release of metabolites from probiotic microorganisms into the extracellular matrix.
Yeast cells are essential to maintain the integrity and viability of microbial populations in kefir drinks.2 They release metabolic byproducts (e.g. amino acids, vitamins, and other compounds) that are necessary for the growth of bacteria, while the metabolic end-products of bacteria can be used by yeast as an energy source.10 Moreover, yeast cells are sensitive to the availability of nutrients and undergo metabolic changes in the time scale of a few hours.11,12 Therefore, one may hypothesise that yeast have a strong influence on the chemical composition of kefir drinks at room temperature. In order to verify this hypothesis, here, we study changes in the relative content of volatile compounds in such probiotic drinks as well as yeast cultures. Volatile secondary metabolites are characterised by high diffusivity and are relatively easy to analyse; hence, they may be considered a convenient indicator of metabolic remodelling of microbial cell-rich matrices. They also have a high impact on organoleptic properties of probiotic foodstuffs. Therefore, in this study, we specifically focused on the changes in the volatomes of probiotic drinks. To reproduce close-to-real conditions, we focused on pre-fermented kefir (i.e. after production and bottling) and incubated such samples at an elevated temperature to induce further (secondary) fermentation. To investigate the dynamics of metabolic processes conducted by microbial strains and involving volatile extracellular metabolites, we implemented gas chromatography (GC) coupled with mass spectrometry (MS) in conjunction with headspace-solid-phase microextraction (HS-SPME). Other analytical techniques were implemented in a comparative pilot study focused on non-volatile metabolites and presented in ESI.†
2. Experimental section
2.1. Chemicals
9-Aminoacridine, 13C10-adenosine triphosphate (13C10-ATP), methanol (LC-MS grade), water (LC-MS grade), ethyl acetate, octyl acetate, phenethyl acetate, hexyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl butyrate, propyl butyrate, butyl butyrate, limonene, terpinene, linalool, glucose assay kit, and acid-washed glass beads were purchased from Sigma-Aldrich (St Louis, MO, USA). An ethanol quantitation kit (Amplite) was purchased from AAT Bioquest (Sunnyvale, CA, USA). Isopentyl acetate was purchased from Alfa Aesar (Heysham, UK). Phenethyl alcohol was purchased from Acros Organics (Fair Lawn, NJ, USA). Yeast mould (YM) broth (Difco) was purchased from Becton Dickinson & Company (Sparks, MD, USA).2.2. Samples and microbial cultures
Five-milliliter aliquots of samples were loaded into 20 mL glass vials (20-HSV; Thermo Fisher Scientific, Waltham, MA, USA) covered with septum caps (18-MSL-ST3; Thermo Fisher Scientific). They were subsequently incubated at 28 °C. Baker's yeast, i.e. Saccharomyces cerevisiae (ATCC 834; BCRC number 20577; Bioresource Collection and Research Center, Hsinchu, Taiwan) were grown in Difco YM broth [0.3% (w/v) yeast extract, 0.3% malt extract, 0.5% peptone, and 1% glucose] at 28 °C. Probiotic drinks, kefir (ingredients: milk, kefir grains; non-fat solids >8.2%, butter oil 3.0–3.8%) and dessert yogurt (ingredients: water, milk powder, sucrose, pectin, flavour additive, galactooligosaccharide, Bifidobacterium lactis Bb12, Lactobacillus acidophilus La5, Streptococcus thermophilus and Lactobacillus bulgaricus), were purchased from local shops (Hsinchu, Taiwan). The original bottles were refrigerated at ∼4 °C until the start of the experiment.2.3. Analysis of volatile secondary metabolites by GC-MS
The monitoring of metabolic processes of microorganisms during secondary fermentation was performed according to the scheme in Fig. 1. A manual SPME fibre assembly [divenylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), Stableflex, cat. no. 57328-U; Supelco, Bellefonte, PA, USA] was used to extract volatile extracellular metabolites from the headspace of the culture vials, which was then followed by GC-MS analysis. This way of sampling/analysis is very convenient because one does not need to open the vial to withdraw an aliquot of liquid suspension for analysis. Therefore, perturbation to the microbial cell culture is kept to a minimum. Moreover, HS-SPME greatly reduces matrix effects, which is particularly important when analyzing real samples such as liquid cell cultures and dairy products. The extraction time and temperature used in the SPME method were selected following preliminary experiments. New SPME fibres were conditioned at 270 °C for 60 min, as recommended by the manufacturer. Prior to analysis, SPME fibres were incubated in the injector of the GC system at 250 °C for 15 min and then cooled down at room temperature for 5 min. The SPME device was inserted into the sealed vial by manually penetrating the septum, and the fibre was subsequently exposed to the headspace of the sample vial for 5 min. The temperature of 28 °C was maintained during sampling to reduce the occurrence of biological artefacts and to improve reproducibility. After sampling, the SPME fibre was immediately inserted into the GC injector, and the extracted analytes were thermally desorbed at 250 °C for 2 min.2.4. Glucose assay
One-milliliter aliquots of the yeast culture in YM broth were obtained from the 20 mL glass vials and transferred into 2 mL microcentrifuge tubes held on ice. The suspension of cells was then centrifuged at 3300g for 5 min to separate yeast cells from culture medium. The culture medium was kept at −20 °C until analysis. Further, the concentration of glucose was monitored using the glucose assay kit. Briefly, glucose is oxidised to gluconic acid and hydrogen peroxide by glucose oxidase. Then, hydrogen peroxide reacts with o-dianisidine in the presence of peroxidase to form a coloured product (oxidised o-dianisidine). The absorbance of the coloured product (measured at 540 nm) is proportional to the concentration of glucose.2.5. Ethanol assay
The concentration of ethanol in yeast culture medium was monitored using the Amplite ethanol quantitation kit. The samples were treated in the same way as in the glucose assay (Section 2.4). The ethanol assay was based on the oxidation of ethanol by alcohol oxidase. The absorbance of the coloured product (measured at 570 nm) is proportional to the concentration of ethanol.2.6. Instruments
The analysis of volatile extracellular metabolites was performed using a commercial gas chromatograph (TRACE GC; Thermo Fisher Scientific, Waltham, MA, USA) coupled with a single quadrupole mass spectrometer (ISQ). A capillary column with a non-polar phase comprising 5% phenyl methyl polysiloxane (TRACE TR-5; length: 30 m; ID: 0.25 mm; film thickness: 0.25 μm) was used. The eluting compounds were ionised by an electron ionisation (EI) source operating at 70 eV. Internal calibration of the mass spectrometer was conducted using perfluorotributylamine (Alachua, FL, USA) as the calibrant. Helium gas was used as the mobile phase with a constant flow rate of 1 mL min−1. The injector was kept at 250 °C. Splitless injection was used. The column temperature was initially set to 50 °C. Following 2 min of separation, the temperature was gradually increased, reaching 250 °C after 12 min (20 °C min−1 ramp). Then, it was kept constant until the end of the analysis. The mass spectrometer was set to record ions in the m/z range of 30–500 u e−1.2.7. Data treatment
The GC-MS data were acquired using the Xcalibur software (ver. 2.1, 2011, Thermo Fisher Scientific, Waltham, MA, USA). Initial data treatment was carried out in this software; the MS chromatograms were displayed for the m/z range of 80–130 u e−1 and were further designated as partial total ion currents (PTICs). The chromatograms were subsequently exported to the ASCII format and further treated in a separate software program (PeakFit, ver. 4.12, 1999–2003; Seasolve Software, San Jose, CA, USA). A linear function was fitted to the baseline. A fast Fourier transform (FFT) filter was used for smoothing. Haarhoff–Van der Linde (HVL) functions, which provide good representation of asymmetric features, were fitted to all the chromatographic peaks of interest. After the graphical update of fitting, the areas under the peaks were computed. The resulting plots were displayed using OriginPro software (ver. 8, 1991–2007; OriginLab Corporation, Northampton, MA, USA) and finished using CorelDraw software (ver. X5, 2010; Mountain View, CA, USA).The procedure used to identify volatile metabolites was as follows: (i) EI spectra were submitted for search in an electronic library [National Institute of Standards and Technology (NIST)], and candidate compounds (score >80) were selected; (ii) artificial samples containing pure standards of those candidates were analysed using the same GC-MS instruments and methods, and subsequently, retention times as well as EI mass spectra of the target species, recorded for the real samples and the artificial samples, were compared.
3. Results and discussion
Volatile compounds in yogurt and kefir were previously studied using different techniques, such as chromatography,9,13–15 near-infrared (NIR) spectroscopy coupled to an electronic nose,16,17 and proton-transfer-reaction time-of-flight (TOF)-MS.18,19 However, none of those studies focused on the monitoring of volatile extracellular metabolites in kefir in the course of secondary fermentation. In the present work, temporal profiles of extracellular metabolites produced by microorganisms present in probiotic drinks were recorded and compared with the profiles of the corresponding metabolites produced by Saccharomyces cerevisiae cultured on liquid and solid media. This monitoring was enabled by HS-SPME used in conjunction with GC-MS (Fig. 1).3.1. Build-up of volatile extracellular metabolites in probiotic drinks during secondary fermentation
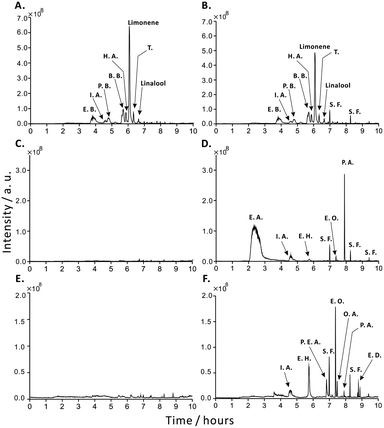
Using GC-MS in conjunction with HS-SPME, it was possible to confirm the presence of a number of volatile extracellular metabolites, which were continuously released by the samples of probiotic drinks of yogurt (Fig. 2B) and kefir (Fig. 2D) during the course of a 24 h incubation at 28 °C. It should be noted that the relative amounts of volatile compounds in yogurt recorded at the beginning of experiment (Fig. 2A) are quite similar to those recorded after the 24 h incubation (Fig. 2B). These compounds are flavour additives used in the food industry (e.g. butyrate, terpene), and their concentrations are not markedly influenced by microbial activity. Interestingly, unlike in the case of yogurt, temporal analysis of kefir reveals ascending profiles of volatile extracellular metabolites, which are related to the activity of live microorganisms (Fig. 2D). Importantly, these volatile extracellular metabolites were not observed at the beginning of incubation (Fig. 2C), although they may be present then at trace concentrations that are below the limits of detection (LODs) of the HS-SPME/GC-MS method. In fact, LODs estimated for these extracellular metabolites range from 1 to 43 μg L−1 (Table S1†). The results mentioned above may lead to a conclusion that the metabolism of microbial cells in kefir is so slow that it takes a few hours to release substantial (detectable) amounts of volatile metabolites.As expected, following a 72 h incubation of yogurt at 28 °C, HS-SPME/GC-MS did not reveal any volatile compounds, which could be linked with the microbial exometabolome/volatome (Fig. S1†). The presence of these compounds may be due to the fact that commercial yogurt products are often supplemented with a number of artificial or natural flavour additives.5,20 In the case of Bifidobacteria, aerobic conditions and the acidic environment inhibit the growth or reduce the activity of cells.21 In fact, the pH of yogurt was ∼4, and it decreased to ∼3 during the 72 h incubation (Fig. S2†). In the following experiment, we performed a long-term (0–72 h) monitoring of volatile extracellular metabolites released by kefir (Fig. 3) and yogurt (Fig. S3†). In this study, it was assumed that the relative abundances of metabolites in the headspace could serve as proxy values for the concentrations of these metabolites in liquid samples (kefir, yogurt, and yeast cultures). Although the sample vials were sealed tight with a silicone/PTFE septum, there always exists a risk of some loss of volatile compounds. We verified this by using an internal standard (thymol). This compound was mixed with samples right before the incubation at 28 °C and monitored by HS-SPME/GC-MS in the same way as done in the case of the sample-related compounds. The results show that the signal of thymol was relatively stable over the entire 72 h period with only moderate declines noticeable for some samples (Fig. 3, S3 and S4†). The profiles of the sample components discussed above have much greater slopes than the one recorded for thymol; therefore, they can be regarded as reliable data.
 | ||
| Fig. 3 HS-SPME/GC-MS analysis applied in the dynamic monitoring of kefir during incubation at 28 °C. The three symbols (square, circle, and triangle) indicate three experimental replicates. | ||
Overall, the above results show considerable dynamics of volatile extracellular metabolites released to the headspace during incubation processes and prove this technique can be used to keep track of alterations to the chemical composition of probiotic drink samples, which are related to the dynamics of the microbial volatome. Notably, the concentrations of all volatile extracellular metabolites slightly decreased over time in the case of yogurt (Fig. S3†). However, in the case of kefir, the levels of most volatile extracellular metabolites increased over time (Fig. 3). This effect was consistently observed in replicate experiments conducted at different times and on different samples of kefir (cf. square, circle, and triangle in Fig. 3).
3.2. Yeast culture as a simple metabolic model of kefir
We used a liquid culture of S. cerevisiae as a simple model of the metabolic processes occurring in kefir during storage at simulated ambient conditions (28 °C). Due to the reduced complexity of the sample matrix, in this case, it was possible to analyse volatile extracellular metabolites (by HS-SPME/GC-MS) as well as some non-volatile species (cf. ESI†). In addition, the GC-MS results point to several compounds, which occur in kefir samples as well as in the yeast culture on liquid and solid medium after 24 h incubation at 28 °C (Fig. 2D and F and S5†). This observation emphasises a significant contribution of yeast to the chemical composition of kefir. The most apparent metabolites occurring in both types of samples include acetate esters (isopentyl acetate and phenethyl acetate) and ethyl esters (ethyl hexanoate, ethyl octanoate, and ethyl decanoate). These esters are related to the metabolism of yeast. In fact, they are formed in the enzymatic process and are responsible for the fruity aroma of yeast cultures.22,23 They may diffuse through the cellular membranes out of the cells and accumulate in the growth medium.22By comparing the dynamics of ethyl esters present in kefir and yeast culture samples (Fig. 3, S4–S6†), one can notice that ethyl esters appeared in kefir after ∼24 h, and their concentrations subsequently increased over time. In the case of the yeast culture, the concentrations of most ethyl esters increased immediately, whereas the concentrations of some of them slightly decreased during the course of incubation at 28 °C. Due to the complexity of the microbial composition and the symbiotic relationships between microbial strains in kefir, the growth and survival of individual species are likely dependent on the presence of other species. Therefore, the biosynthetic rates of esters should be affected not only by the presence of probiotic microorganisms and concentrations of metabolic precursors but also by the influence of symbiotic interactions and the activity of the enzymes involved in synthesis and degradation.23–27 Moreover, the high viscosity of kefir may additionally contribute to the suppression of the release (and/or diffusion) of volatile extracellular metabolites, and it may slow down the uptake of oxygen and nutrients by microorganisms. As the carbohydrate substrate (glucose) is consumed by cells (Fig. 4A), the concentration of extracellular ethanol increased (Fig. 4B). It is known that due to the so-called diauxic shift, cells remodel metabolism and start using ethanol as the growth substrate while producing acetate.11 This not only leads to the acidification of the medium (Fig. S2†), but also results in the inhibition of cell growth (Fig. 4C): although cells can use ethanol as a growth substrate, high concentrations of ethanol eventually inhibit cell growth.
While a pilot study presented in ESI† (cf. Fig. S7 and S8†) also points to the dynamics of selected non-volatile species in kefir and the “reference” S. cerevisiae cultures, further comprehensive analysis using an advanced instrumental analysis toolkit is required to unravel the connections between the volatome and liquid-phase metabolome of kefir.
4. Conclusions
In summary, HS-SPME was used in conjunction with GC-MS to monitor the dynamics of several volatile metabolites in probiotic drinks (in particular, kefir) as well as single-species yeast cultures. According to the above results, the kefir volatome exhibits pronounced dynamics within the time range of tens of hours while stored at ambient conditions. Although the high viscosity and complex composition of probiotic drinks may suppress the release of volatile extracellular metabolites and slow down the metabolism of microorganisms, kefir and S. cerevisiae cultures show similar temporal volatome profiles. Therefore, such single-species cultures can serve, to some extent, as models for the metabolism of probiotic microorganisms found in certain dairy products such as kefir. However, one big disadvantage of this model is the inability to mimic symbiotic interactions between the yeast and bacterial cells.Acknowledgements
We acknowledge the National Science Council of Taiwan for the financial support.Notes and references
- R. Fuller, J. Appl. Microbiol., 1989, 66, 365 CrossRef CAS PubMed.
- E. R. Farnworth, Handbook of Fermented Functional Foods, CRC Press, Boca Raton, 2nd edn, 2008 Search PubMed.
- H. S. Gill and F. Guarner, Postgrad. Med. J., 2004, 80, 516 CrossRef CAS PubMed.
- O. Adolfsson, S. N. Meydani and R. M. Russell, Am. J. Clin. Nutr., 2004, 80, 245 CAS.
- A. Y. Tamime and R. K. Robinson, Yogurt Science and Technology, CRC Press, Boca Raton, 2nd edn, 1999 Search PubMed.
- H. Cheng, Crit. Rev. Food Sci. Nutr., 2010, 50, 938 CrossRef CAS PubMed.
- Z. B. Guzel-Seydim, T. Kok-Tas, A. K. Greene and A. C. Seydim, Crit. Rev. Food Sci. Nutr., 2011, 51, 261 CrossRef CAS PubMed.
- H. S. Kwak, S. K. Park and D. S. Kim, J. Dairy Sci., 1996, 79, 937 CrossRef CAS.
- C. Condurso, A. Verzera, V. Romeo, M. Ziino and F. Conte, Int. Dairy J., 2008, 18, 819 CrossRef CAS PubMed.
- B. C. Viljoen, Int. J. Food Microbiol., 2001, 69, 37 CrossRef CAS.
- G. G. Zampar, A. Kűmmel, J. Ewald, S. Jol, B. Niebel, P. Picotti, R. Aebersold, U. Sauer, N. Zamboni and M. Heinemann, Mol. Syst. Biol., 2013, 9, 651 CrossRef PubMed.
- J. Pátková, D. Šmogrovičová, P. Bafrncová and Z. Dőmĕny, Folia Microbiol., 2000, 45, 335 CrossRef.
- M. Ligor, R. Jarmalaviciene, M. Szumski, A. Maruška and B. Buszewski, J. Sep. Sci., 2008, 31, 2707 CrossRef CAS PubMed.
- A. Aghlara, S. Mustafa, Y. A. Manap and R. Mohamad, Int. J. Food Prop., 2009, 12, 808 CrossRef CAS.
- A. Irigoyen, M. Ortigosa, S. García, F. C. Ibánez and P. Torre, Int. J. Dairy Technol., 2012, 65, 578 CrossRef PubMed.
- C. Cimander, M. Carlsson and C. F. Mandenius, J. Biotechnol., 2002, 99, 237 CrossRef CAS.
- M. Narvátil, C. Cimander and C. F. Mandenius, J. Agric. Food Chem., 2004, 52, 415 CrossRef PubMed.
- C. Soukoulis, E. Aprea, F. Biasioli, L. Cappellin, E. Schuhfried, T. Märk and F. Gasperi, Rapid Commun. Mass Spectrom., 2010, 24, 2127 CrossRef CAS PubMed.
- C. Soukoulis, F. Biasioli, E. Aprea, E. Schuhfried, L. Cappellin, T. D. Märk and F. Gasperi, Food Bioprocess Technol., 2012, 5, 2085 CrossRef CAS.
- C. G. Vinderola, G. A. Costa, S. Regenhardt and J. A. Reinheimer, Int. Dairy J., 2002, 12, 579 CrossRef CAS.
- T. D. Boylston, C. G. Vinderola, H. B. Ghoddusi and J. A. Reinheimer, Int. Dairy J., 2004, 14, 375 CrossRef CAS PubMed.
- S. Q. Liu, R. Holland and V. L. Crow, Int. Dairy J., 2004, 14, 923 CrossRef CAS PubMed.
- S. M. G. Saerens, F. Delvaux, K. J. Verstrepen, P. Van Dijck, J. M. Thevelein and F. R. Delvaux, Appl. Environ. Microbiol., 2008, 74, 454 CrossRef CAS PubMed.
- S. Procopio, F. Qian and T. Becker, Eur. Food Res. Technol., 2011, 233, 721 CrossRef CAS PubMed.
- G. Styger, B. Prior and F. F. Bauer, J. Ind. Microbiol. Biotechnol., 2011, 38, 1145 CrossRef CAS PubMed.
- S. M. G. Saerens, P. J. Verbelen, N. Vanbeneden, J. M. Thevelein and F. R. Delvaux, Appl. Microbiol. Biotechnol., 2008, 80, 1039 CrossRef CAS PubMed.
- S. M. G. Saerens, K. J. Verstrepen, S. D. M. Van Laere, A. R. D. Voet, P. V. Dijck, F. R. Delvaux and J. M. Thevelein, J. Biol. Chem., 2006, 281, 4446 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental details, results and discussion, table, and figures. See DOI: 10.1039/c4ra02990a |
| ‡ S.G. is currently based in Sirimapura, Rajanganaya, Sri Lanka. |
| This journal is © The Royal Society of Chemistry 2014 |