Fabrication of FeF3 nanocrystals dispersed into a porous carbon matrix as a high performance cathode material for lithium ion batteries
Abstract
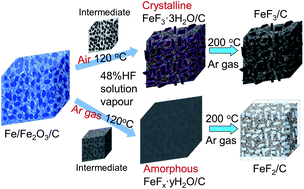
FeF3/C nanocomposites, where FeF3 nanocrystals had been dispersed into a porous carbon matrix, were successfully fabricated by a novel vapour–solid method in a tailored autoclave. Phase evolution of the reaction between the precursor and HF solution vapour under air and argon gas atmospheres were investigated. The results showed that the air in the autoclave played an important role in driving the reaction to form FeF3. The as-prepared FeF3/C delivered 134.3, 103.2 and 71.0 mA h g−1 of charge capacity at a current density of 104, 520, and 1040 mA g−1 in turn, exhibiting superior rate capability to the bare FeF3. Moreover, it displayed stable cycling performance, with a charge capacity of 196.3 mA h g−1 at 20.8 mA g−1. EIS and BET investigations indicated that the good electrochemical performance can be attributed to the good electrical conductivity and high specific surface area that result from the porous carbon matrix.

 Please wait while we load your content...
Please wait while we load your content...