Surfactantco-assembly and ion exchange to modulate polyelectrolyte multilayer wettability†
Abstract
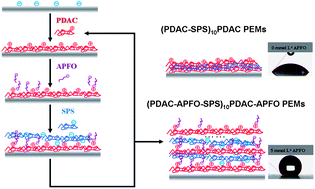
Coatings with controllable wettability are of interest in a number of applications. Modulation of surface wettability is often necessary for these applications. It has been demonstrated many times that surface topography is key to the ability to make superhydrophobic surfaces, but at the same time surface chemistry is key. The layer-by-layer technique of fabricating coatings based on the complexation of oppositely charged polyelectrolytes can be a versatile method for producing precisely controlled thin films with a wide range of properties. In order to control wettability, hierarchical textures are generally needed, but the underlying surface energy of the material is also important. Generally, these films are made out of commercially available

 Please wait while we load your content...
Please wait while we load your content...