
RSC Advances
Subject area
Organic articles published in the last
6 months

New multiple-layered 3D polymers showing aggregation-induced emission and polarization
An exceptional achiral and chiral multilayer 3D polymer has been created and controlled by uniform and distinct aromatic chromophore units that are multiply sandwiched by naphthyl berths.

RSC Adv., 2024,14, 13342-13350
https://doi.org/10.1039/D4RA02128B
Synthesis of novel phthalazine-based derivatives with potent cytotoxicity against HCT-116 cells through apoptosis and VEGFR2 inhibition
A novel phthalazine derivative exhibited potent cytotoxicity against HCT-116 cells as VEGFR2 inhibitor and apoptosis cell death.

RSC Adv., 2024,14, 13027-13043
https://doi.org/10.1039/D4RA02103G
Mono and di ortho-C–H acetoxylation of 2-aryloxyquinoline-3-carbaldehydes
A novel protocol for introducing an acetoxy functional group selectively on the ortho aryl sp2 carbon of 2-aryloxyquinoline-3-carbaldehydes in good yields with good functional group tolerance using a palladium catalyst was developed for the first time.
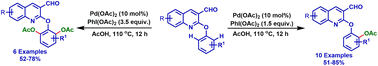
RSC Adv., 2024,14, 13306-13310
https://doi.org/10.1039/D4RA01289E
Synthesis molecular docking and DFT studies on novel indazole derivatives
3-Carboxamide indazoles have been developed using the amide coupling process. Density function theory (DFT) computations, and the assessment of binding energy using Auto Dock, illustrate the pharmaceutical effectiveness.
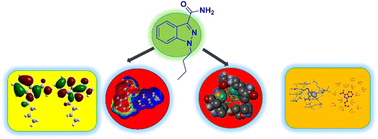
RSC Adv., 2024,14, 13218-13226
https://doi.org/10.1039/D4RA02151G
Grind, shine and detect: mechanochemical synthesis of AIE-active polyaromatic amide and its application as molecular receptor of monovalent anions or nucleotides
The mechanochemical synthesis of AIE-active amide, as well as the application of the title compound as molecular receptor of monovalent anions and nucleotides, are described.

RSC Adv., 2024,14, 13227-13236
https://doi.org/10.1039/D4RA02129K
Regioselective C(sp2)–H halogenation of pyrazolo[1,5-a]pyrimidines facilitated by hypervalent iodine(III) under aqueous and ambient conditions
An efficient and mild approach has been developed for the regio-selective direct C3 halogenation of pyrazolo[1,5-a]pyrimidines employing readily available potassium halide salts and a hypervalent iodine(III) reagent at ambient temperature.
![Graphical abstract: Regioselective C(sp2)–H halogenation of pyrazolo[1,5-a]pyrimidines facilitated by hypervalent iodine(iii) under aqueous and ambient conditions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4RA02090A&imageInfo.ImageIdentifier.Year=2024)
RSC Adv., 2024,14, 13095-13099
https://doi.org/10.1039/D4RA02090A
Exploring the synthetic potential of epoxide ring opening reactions toward the synthesis of alkaloids and terpenoids: a review
Epoxides are oxygen containing three-membered heterocycles which can undergo ring opening reactions. These reactions have been significantly employed in the synthesis of alkaloids and terpenoids-based natural products.

RSC Adv., 2024,14, 13100-13128
https://doi.org/10.1039/D4RA01834F
The conjugates of 5′-deoxy-5-fluorocytidine and hydroxycinnamic acids – synthesis, anti-pancreatic cancer activity and molecular docking studies
New conjugates 1–6 containing 5-dFCR and selected hydroxycinnamic acids were synthesized and tested in vitro against pancreatic cancer (PDAC) lines. The ADME properties and molecular docking to CES2 or human albumin were discussed.

RSC Adv., 2024,14, 13129-13141
https://doi.org/10.1039/D4RA01683A
Development of an imidazole-based N,N-bidentate ligand for the manganese catalyzed direct coupling of nitriles with alcohols
This work describes the manganese catalyzed direct coupling of nitriles with alcohols with assistance of a set of simple and new imidazole-based N,N-bidentate ligands.

RSC Adv., 2024,14, 12978-12982
https://doi.org/10.1039/D4RA00817K
Nickel-catalyzed γ-alkylation of cyclopropyl ketones with unactivated primary alkyl chlorides: balancing reactivity and selectivity via halide exchange
Nickel-catalyzed cross-electrophile coupling of cyclopropyl ketones and alkyl chlorides. High reactivity and selectivity can be achieved with sodium iodide as a cocatalyst that generates a low concentration of alkyl iodide via halide exchange.

RSC Adv., 2024,14, 12883-12887
https://doi.org/10.1039/D4RA02616K
The untold story of starch as a catalyst for organic reactions
Starch as catalyst for organic reactions.

RSC Adv., 2024,14, 12676-12702
https://doi.org/10.1039/D4RA00775A
Ethyl acetate as an acetyl source and solvent for acetylation of alcohols by KOH
A mild, efficient and convenient procedure for the acetylation of alcohols with EtOAc as acetyl source and solvent mediated by KOH was reported.

RSC Adv., 2024,14, 12574-12579
https://doi.org/10.1039/D3RA08717D
Electrochemical oxidative radical cascade cyclization of dienes and diselenides towards the synthesis of seleno-benzazepines
An electrochemical approach to access seleno-benzazepines through an oxidative radical cascade cyclization of dienes with diselenides under metal-free, external oxidant-free and base-free conditions.

RSC Adv., 2024,14, 12556-12560
https://doi.org/10.1039/D4RA01914H
Visible light mediated iron-catalyzed addition of oxamic acids to imines
Oxamic acids where shown to add to imines, providing a broad range of α-aminoacid amides in generally good yields.

RSC Adv., 2024,14, 12528-12532
https://doi.org/10.1039/D4RA02258K
Synthesis and study of antibiofilm and antivirulence properties of flavonol analogues generated by palladium catalyzed ligand free Suzuki–Miyaura coupling against Pseudomonas aeruginosa PAO1
An overview on the mechanism of Pseudomonas aeruginosa PAO1 biofilm inhibition by Suzuki–Miyaura coupled flavonols.

RSC Adv., 2024,14, 12278-12293
https://doi.org/10.1039/D3RA08617H
LED-induced Ru-photoredox Pd-catalyzed C–H arylation of (6-phenylpyridin-2-yl)pyrimidines and heteroaryl counterparts
A LED-induced Ru-photoredox Pd-catalyzed method was employed to carry out late-stage C–H arylation on a series of Biginelli/Suzuki-derived modular (6-phenylpyridin-2-yl)pyrimidine substrates and their heteroaryl counterparts.

RSC Adv., 2024,14, 12179-12191
https://doi.org/10.1039/D4RA02173H
Bicyclic guanidine superbase carboxylate salts for cellulose dissolution
Bicyclic guanidines are utilized in organic synthesis as base catalysts or reagents.

RSC Adv., 2024,14, 12119-12124
https://doi.org/10.1039/D4RA01734J
Redox-neutral α-functionalization of pyrrolidines: facile access to α-aryl-substituted pyrrolidines
Using a quinone monoacetal as the oxidant and DABCO as the base, we report the one-step synthesis of α-aryl-substituted pyrrolidines from pyrrolidine. The reaction of pyrrolidine and quinone monoacetal in 2,2,2-trifluoroethanol afforded octahydro-dipyrroloquinoline in high yield.

RSC Adv., 2024,14, 11986-11991
https://doi.org/10.1039/D4RA00983E
Keto–enol equilibrium: stable tautomers of ortho-, meta-, and para-hydroquinones in large aromatics
Keto–enol tautomerism of hydroquinones was studied for fused aromatic compounds. For larger fused aromatic compounds the dione tautomer was the stable form. These experimental results were corroborated by calculations.
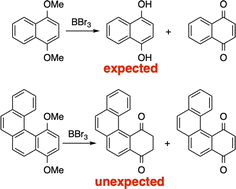
RSC Adv., 2024,14, 11969-11976
https://doi.org/10.1039/D4RA02202E
Recent advances in the transformation reactions of the Betti base derivatives
This review article highlights the use of Betti base derivatives in transformation reactions for synthesizing organic compounds, particularly heterocyclic molecules, and investigation into their pharmacological properties.

RSC Adv., 2024,14, 11811-11848
https://doi.org/10.1039/D4RA01256A
U-shaped stereoscopic design strategy toward N-doped nanographene segment
Through a stereoscopic design strategy, U-shaped N-heteroacene was synthesized, and shows regular and incompletely closed pores within the crystal structure.

RSC Adv., 2024,14, 11771-11774
https://doi.org/10.1039/D4RA00788C
Activity against Mycobacterium tuberculosis of a new class of spirooxindolopyrrolidine embedded chromanone hybrid heterocycles
Spirooxindolopyrrolidines were synthesized in quantitative yield through cycloaddition strategy. Compounds exhibited significant anti-tubercular activity and molecular docking studies of the compound is well correlates with in vitro findings.

RSC Adv., 2024,14, 11604-11613
https://doi.org/10.1039/D4RA01501K
A simple protocol for the synthesis of perylene bisimides from perylene tetracarboxylic dianhydride
PBIs were synthesized from the reaction of perylene bisanhydrides and primary amines in imidazole as solvent. Maleimide, anthraquinone or ferrocene were bonded to the side chain and the S-trityl group was replaced by imidazole in S-trityl cysteine.

RSC Adv., 2024,14, 11141-11150
https://doi.org/10.1039/D4RA01576B
Synthesis of novel bioactive pyrido[2,3-d]pyrimidine derivatives with potent cytotoxicity through apoptosis as PIM-1 kinase inhibitors
Novel bioactive pyrido[2,3-d]pyrimidine derivatives with potent apoptotic inducers as PIM-1 kinase inhibitors.
![Graphical abstract: Synthesis of novel bioactive pyrido[2,3-d]pyrimidine derivatives with potent cytotoxicity through apoptosis as PIM-1 kinase inhibitors](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4RA00902A&imageInfo.ImageIdentifier.Year=2024)
RSC Adv., 2024,14, 11098-11111
https://doi.org/10.1039/D4RA00902A
Continuous flow biocatalysis: synthesis of purine nucleoside esters catalyzed by lipase TL IM from Thermomyces lanuginosus
Purine nucleoside ester is one of the derivatives of purine nucleoside, which has antiviral and anticancer activities.

RSC Adv., 2024,14, 10953-10961
https://doi.org/10.1039/D4RA00097H
Browse by Subject
- All (337 articles)
- Atomic/elemental (15 articles)
- Bioanalytical (69 articles)
- Chemometrics (31 articles)
- Crystallography (167 articles)
- Electroanalytical (59 articles)
- Imaging/microscopy (33 articles)
- Mass spectrometry (25 articles)
- Medical diagnostics (81 articles)
- Microfluidics (15 articles)
- Nanoanalysis (21 articles)
- Separation science (27 articles)
- Spectroscopy (94 articles)
- All (271 articles)
- Bioinorganic chemistry (52 articles)
- Bioorganic chemistry (29 articles)
- Biotechnology (44 articles)
- Cellular chemistry (59 articles)
- Computational (88 articles)
- Drug delivery (73 articles)
- Drug discovery (188 articles)
- Imaging/diagnostics (14 articles)
- Molecular biology (6 articles)
- Nanotechnology (40 articles)
- Natural products (30 articles)
- Pharmacology (96 articles)
- Photobiology (15 articles)
- Polymorphism (pharma) (1 article)
- Structural biology (12 articles)
- Toxicology (3 articles)
- All (551 articles)
- Biomaterials (101 articles)
- Biopolymers (97 articles)
- Carbon materials (101 articles)
- Composites (240 articles)
- Electronic materials (91 articles)
- Encapsulation (1 article)
- Energy applications (81 articles)
- Films/membranes (80 articles)
- Gels & soft matter (33 articles)
- Inorganic materials (49 articles)
- Medical materials (63 articles)
- Nanomaterials (125 articles)
- Optical materials (50 articles)
- Organic materials (50 articles)
- Polymers (121 articles)
- All (340 articles)
- Assembly (9 articles)
- Biotechnology (29 articles)
- Carbon nanomaterials (81 articles)
- Imaging/microscopy (15 articles)
- Nanoanalysis (38 articles)
- Nanocatalysis (33 articles)
- Nanomaterials (216 articles)
- Nanomedicine (39 articles)
- Nanotoxicology (50 articles)
- Optical nanomaterials (33 articles)
- Synthesis (12 articles)
- All (183 articles)
- Bioorganic (18 articles)
- Catalysis (46 articles)
- Fine chemicals (27 articles)
- Natural products (25 articles)
- Physical organic (8 articles)
- Stereochemistry (11 articles)
- Supramolecular (3 articles)
- Sustainable synthesis (36 articles)
- Synthetic methodology (107 articles)
- Total synthesis (14 articles)
- All (331 articles)
- Biophysics (2 articles)
- Charge transfer (30 articles)
- Electrochemistry (12 articles)
- Energy research (19 articles)
- Imaging/microscopy (6 articles)
- Kinetics & dynamics (43 articles)
- Materials (107 articles)
- Mechanics (34 articles)
- Nanoscience (71 articles)
- Photoscience (27 articles)
- Quantum & theoretical (94 articles)
- Simulations (23 articles)
- Single molecules (45 articles)
- Soft matter (5 articles)
- Spectroscopy (7 articles)
- Surfaces & interfaces (73 articles)