
RSC Advances
Subject area
Stereochemistry articles published in the last
6 months

Recent progress and prospects in the organocatalytic Morita–Baylis–Hillman reaction
The organocatalytic Morita–Baylis–Hillman (MBH) reaction, one of the most significant carbon–carbon bond formations involves the addition of α,β-unsaturated carbonyl compounds to activated alkenes to give α-methylene-β-hydroxycarbonyl compounds.

RSC Adv., 2024,14, 14949-14963
https://doi.org/10.1039/D4RA02369B
A green bio-organic catalyst (taurine) promoted one-pot synthesis of (R/S)-2-thioxo-3,4-dihydropyrimidine(TDHPM)-5-carboxanilides: chiral investigations using circular dichroism and validation by computational approaches
Twenty-three new derivatives of (R/S)-TDHPM-5-carboxanilide have been synthesized with up to 99% yield. All racemates were separated using chiral HPLC (Prep LC) which provided up to 99.99% purity. AC was determined using circular dichroism spectra.

RSC Adv., 2024,14, 9300-9313
https://doi.org/10.1039/D4RA01391C
Circularly polarized luminescence and high photoluminescence quantum yields from rigid 5,10-dihydroindeno[2,1-a]indene and 2,2′-dialkoxy-1,1′-binaphthyl conjugates and copolymers
Rigid planar carbon-bridged oligo(phenylenevinylene)–binaphthyl conjugates and alternating copolymers exhibit high luminescence quantum yield of >0.95, along with circular polarized luminescence dissymmetry factors of up to 1.1 × 10−3.
![Graphical abstract: Circularly polarized luminescence and high photoluminescence quantum yields from rigid 5,10-dihydroindeno[2,1-a]indene and 2,2′-dialkoxy-1,1′-binaphthyl conjugates and copolymers](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4RA00380B&imageInfo.ImageIdentifier.Year=2024)
RSC Adv., 2024,14, 7251-7257
https://doi.org/10.1039/D4RA00380B
Hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone to chiral tropic acid
The hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone using a chiral PTC and water-free basic anion exchange resin as the hydroxide ion donor was achieved.
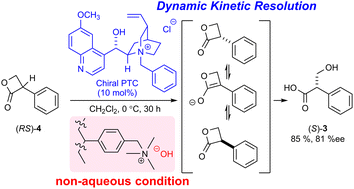
RSC Adv., 2024,14, 6121-6126
https://doi.org/10.1039/D3RA08594E
Enantioselective synthesis of [1,1′-binaphthalene]-8,8′-diyl bis(diphenylphosphane) and its derivatives
A new chiral phosphine ligand and its diol derivative have been designed and synthesized asymmetrically.
![Graphical abstract: Enantioselective synthesis of [1,1′-binaphthalene]-8,8′-diyl bis(diphenylphosphane) and its derivatives](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3RA07956B&imageInfo.ImageIdentifier.Year=2024)
RSC Adv., 2024,14, 2792-2795
https://doi.org/10.1039/D3RA07956B
Retraction: Enantioselective synthesis of α-tetrasubstituted (3-indolizinyl) (diaryl)methanamines via chiral phosphoric acid catalysis
RSC Adv., 2024,14, 1888-1888
https://doi.org/10.1039/D4RA90001D
Stereoselectivity in electrosprayed confined volumes: asymmetric synthesis of warfarin by diamine organocatalysts in microdroplets and thin films
Asymmetric synthesis of warfarin in ESI-microdroplets by using chiral 1,2-diamino organocatalysts.

RSC Adv., 2024,14, 1576-1580
https://doi.org/10.1039/D3RA07975A
Enantioselective synthesis of α-tetrasubstituted (1-indolizinyl) (diaryl)-methanamines via chiral phosphoric acid catalysis
Chiral phosphoric acid-catalyzed enantioselective synthesis of α-tetrasubstituted (1-indolizinyl) (diaryl)-methanamines has been developed.
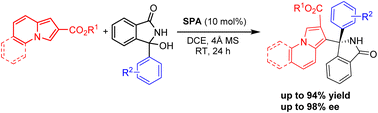
RSC Adv., 2024,14, 1106-1113
https://doi.org/10.1039/D3RA07636A
Intermolecular exo-selective Diels–Alder reaction catalysed by dual-functional Brønsted acid: conformational restriction of transition states by hydrogen bonds as the key interaction
An exo-selective Diels–Alder reaction was predicted by a computational approach and was experimentally confirmed by the combined use of a dual-functional acid catalyst, such as phosphoric acid, and the conformationally restricted dienylcarbamate.

RSC Adv., 2023,13, 36293-36300
https://doi.org/10.1039/D3RA07688A
Synthesis of P-stereogenic 1-phosphanorbornane-derived phosphine–phosphite ligands and application in asymmetric catalysis
A convenient synthesis of enantiopure mixed donor phosphine–phosphite ligands has been developed incorporating P-stereogenic phosphanorbornane and axially chiral bisnaphthols into one ligand structure.

RSC Adv., 2023,13, 34439-34444
https://doi.org/10.1039/D3RA07630J
Asymmetric total synthesis strategies of halichlorine and pinnaic acid
Halichlorine and pinnaic acids are structurally related to natural alkaloids isolated from different marine organisms. This review summarizes the asymmetric synthesis strategies of halichlorine and pinnaic acid using a 6-azaspiro[4.5]decane skeleton as the key intermediate.

RSC Adv., 2023,13, 33754-33769
https://doi.org/10.1039/D3RA06955A
Browse by Subject
- All (336 articles)
- Atomic/elemental (14 articles)
- Bioanalytical (65 articles)
- Chemometrics (30 articles)
- Crystallography (163 articles)
- Electroanalytical (55 articles)
- Imaging/microscopy (32 articles)
- Mass spectrometry (20 articles)
- Medical diagnostics (77 articles)
- Microfluidics (17 articles)
- Nanoanalysis (21 articles)
- Separation science (26 articles)
- Spectroscopy (95 articles)
- All (278 articles)
- Bioinorganic chemistry (50 articles)
- Bioorganic chemistry (27 articles)
- Biotechnology (53 articles)
- Cellular chemistry (56 articles)
- Computational (85 articles)
- Drug delivery (79 articles)
- Drug discovery (186 articles)
- Imaging/diagnostics (16 articles)
- Molecular biology (6 articles)
- Nanotechnology (46 articles)
- Natural products (29 articles)
- Pharmacology (88 articles)
- Photobiology (15 articles)
- Polymorphism (pharma) (1 article)
- Structural biology (14 articles)
- Toxicology (5 articles)
- All (559 articles)
- Biomaterials (102 articles)
- Biopolymers (99 articles)
- Carbon materials (100 articles)
- Composites (248 articles)
- Electronic materials (96 articles)
- Encapsulation (1 article)
- Energy applications (85 articles)
- Films/membranes (83 articles)
- Gels & soft matter (36 articles)
- Inorganic materials (49 articles)
- Medical materials (66 articles)
- Nanomaterials (124 articles)
- Optical materials (52 articles)
- Organic materials (57 articles)
- Polymers (133 articles)
- All (352 articles)
- Assembly (11 articles)
- Biotechnology (31 articles)
- Carbon nanomaterials (80 articles)
- Imaging/microscopy (18 articles)
- Nanoanalysis (43 articles)
- Nanocatalysis (33 articles)
- Nanomaterials (215 articles)
- Nanomedicine (44 articles)
- Nanotoxicology (58 articles)
- Optical nanomaterials (36 articles)
- Synthesis (11 articles)
- All (173 articles)
- Bioorganic (17 articles)
- Catalysis (46 articles)
- Fine chemicals (25 articles)
- Natural products (22 articles)
- Physical organic (8 articles)
- Stereochemistry (11 articles)
- Supramolecular (3 articles)
- Sustainable synthesis (34 articles)
- Synthetic methodology (101 articles)
- Total synthesis (13 articles)
- All (327 articles)
- Biophysics (3 articles)
- Charge transfer (30 articles)
- Electrochemistry (13 articles)
- Energy research (19 articles)
- Imaging/microscopy (5 articles)
- Kinetics & dynamics (42 articles)
- Materials (109 articles)
- Mechanics (34 articles)
- Nanoscience (73 articles)
- Photoscience (26 articles)
- Quantum & theoretical (92 articles)
- Simulations (23 articles)
- Single molecules (41 articles)
- Soft matter (5 articles)
- Spectroscopy (7 articles)
- Surfaces & interfaces (70 articles)