Themed collection Prolines

Applications and future of ion mobility mass spectrometry in structural biology
Ion mobility coupled to mass spectrometry (IMMS) separates gaseous ions on the basis of mass and structural properties. Hence, structural and dynamic information can be obtained for a wide variety of biomacromolecules.

New J. Chem., 2013,37, 1283-1289
https://doi.org/10.1039/C3NJ41051J
Homooligomers of substituted prolines and β-prolines: syntheses and secondary structure investigation
Homooligomers of substituted prolines and β-prolines were synthesized and studied using CD and NMR in water and alcohols.
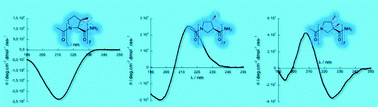
New J. Chem., 2013,37, 1312-1319
https://doi.org/10.1039/C3NJ00127J
Conformational properties of peptides incorporating a fluorinated pseudoproline residue
Structural features of peptides incorporating one CF3-ΨPro residue were analyzed. A type-VI β-turn was observed in a short pseudotetrapeptide sequence.
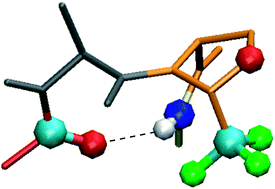
New J. Chem., 2013,37, 1336-1342
https://doi.org/10.1039/C3NJ41084F
Effect of proline analogues on the conformation of elastin peptides
Three elastin model peptides containing the repetitive motif –VGVXGVG–, where X corresponds to (2S)-proline (Pro), (2S,4R)-4-hydroxy-proline (Hyp) and (2S,4R)-4-methoxy-proline (Mop), were synthesized. The peptides were investigated at molecular and supramolecular levels.
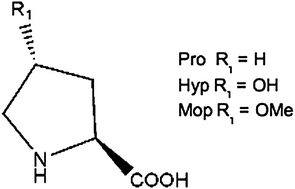
New J. Chem., 2013,37, 1326-1335
https://doi.org/10.1039/C3NJ41001C
Straightforward asymmetric synthesis of Ala-Ψ[CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH]-Pro, a
CH]-Pro, a proline -containing pseudodipeptide bearing a fluoroolefin as a peptide bond mimic
A new efficient asymmetric synthesis has been developed to obtain the relevant fluorinated pseudodipeptide Ala-Ψ[(Z)CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH]-Pro.
CH]-Pro.
![Graphical abstract: Straightforward asymmetric synthesis of Ala-Ψ[CF [[double bond, length as m-dash]] CH]-Pro, a proline-containing pseudodipeptide bearing a fluoroolefin as a peptide bond mimic](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2NJ40891K&imageInfo.ImageIdentifier.Year=2013)
New J. Chem., 2013,37, 1320-1325
https://doi.org/10.1039/C2NJ40891K
About this collection
This themed collection in NJC brings an update on several issues surrounding the potential for prolines and related peptides. It aims to highlight the latest developments in the synthesis and structural elucidation of prolines and derivatives, as well as their biological activities.